m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03499
|
[1], [2] | |||
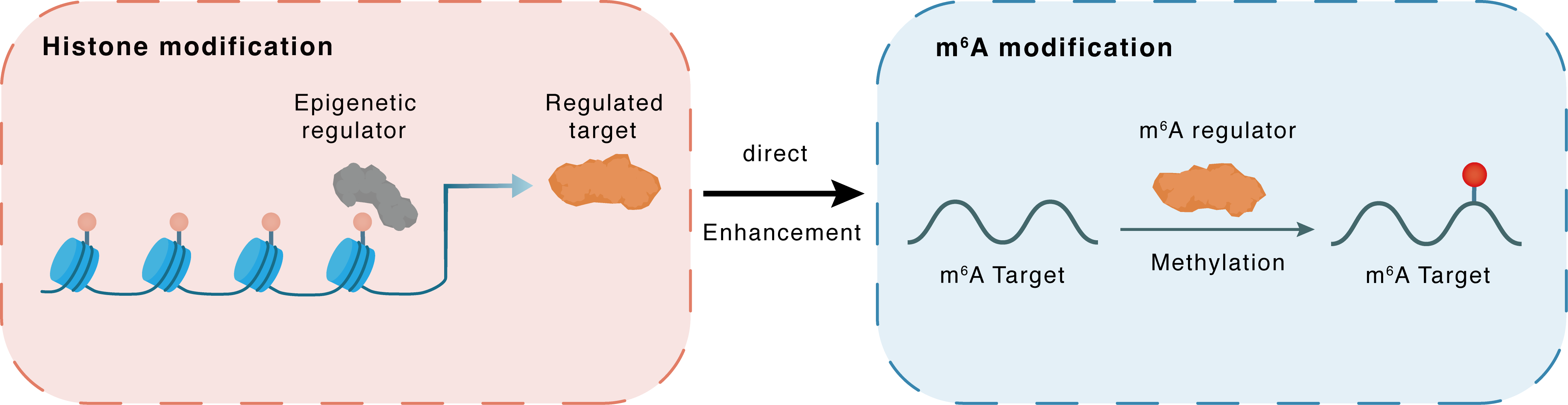 Histone modification
H3K18la
Epigenetic Regulator
METTL14
Direct
Enhancement
m6A modification
AKT1
AKT1
METTL14
Methylation
Histone modification
H3K18la
Epigenetic Regulator
METTL14
Direct
Enhancement
m6A modification
AKT1
AKT1
METTL14
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 14 (METTL14) | WRITER | |||
| m6A Target | RAC-alpha serine/threonine-protein kinase (AKT1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Regulated Target | Histone H3 lysine 18 lactylation (H3K18la) | View Details | |||
| Downstream Gene | METTL14 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | Histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | Histone H3 lysine 18 lactylation (H3K18la) was found to upregulate METTL14 expression. In conclusion, METTL14 knockdown promotes stemness in GC by mediating m6A modification of ATF5 mRNA, which activates the WDR74/beta-catenin axis, making METTL14 a potential therapeutic target for gastric cancer treatment. The m6A modification level was decreased in GC and METTL14 was a key regulator resulting in m6A disorder in GC. METTL14 overexpression suppressed GC cell proliferation and aggression by deactivating the PI3K/RAC-alpha serine/threonine-protein kinase (AKT1)/mTOR pathway and the EMT pathway, respectively. | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
In-vitro Model |
GES-1 | Normal | Homo sapiens | CVCL_EQ22 | |
| SGC-7901 | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| MGC-803 | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| RAC-alpha serine/threonine-protein kinase (AKT1) | 40 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Capivasertib | Approved | [3] | ||
| Synonyms |
1143532-39-1; AZD-5363; capivasertib; AZD 5363; UNII-WFR23M21IE; WFR23M21IE; cc-638; 4-Amino-N-[(1s)-1-(4-Chlorophenyl)-3-Hydroxypropyl]-1-(7h-Pyrrolo[2,3-D]pyrimidin-4-Yl)piperidine-4-Carboxamide; C21H25ClN6O2; (S)-4-AMINO-N-(1-(4-CHLOROPHENYL)-3-HYDROXYPROPYL)-1-(7H-PYRROLO[2,3-D]PYRIMIDIN-4-YL)PIPERIDINE-4-CARBOXAMIDE; 4-Amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-4-piperidinecarboxamide; 4-Piperidinecarboxamide, 4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| GDC-0068 | Phase 3 | [4] | ||
| Synonyms |
RG7440
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 157 nM | |||
| External Link | ||||
| Enzastaurin | Phase 3 | [4] | ||
| Synonyms |
LY317615; LE-0014; LY317615, Enzastaurin; 3-(1-methyl-1H-indol-3-yl)-4-{1-[1-(pyridin-2-ylmethyl)piperidin-4-yl]-1H-indol-3-yl}-1H-pyrrole-2,5-dione; 3-(1-methylindol-3-yl)-4-[1-[1-(pyridin-2-ylmethyl)piperidin-4-yl]indol-3-yl]pyrrole-2,5-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK2110183 | Phase 2 | [5] | ||
| MOA | Modulator | |||
| External Link | ||||
| RX-0201 | Phase 2 | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Trametinib + 2141795 | Phase 2 | [5] | ||
| MOA | Modulator | |||
| External Link | ||||
| PTX-200 | Phase 2 | [4] | ||
| Synonyms |
Plant-derived antiparkinsonian, Phytrix
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CMX-2043 | Phase 2 | [7] | ||
| MOA | Modulator | |||
| External Link | ||||
| ARQ 092 | Phase 2 | [8] | ||
| Synonyms |
Miransertib
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CI-1033 | Phase 2 | [9] | ||
| Synonyms |
Canertinib; Canertinib HCl; Canertinib dihydrochloride; Canertinib dihydrochloride [USAN]; CI1033; PD 183805; Canertinib dihydrochloride (USAN); PD-0183805; PD-183805; Canertinib, PD-183805, CI1033, PD183805; N-[4-(3-Chloro-4-fluorophenylamino)-7-(3-morpholin-4-ylpropoxy)quinazolin-6-yl]acrylamide dihydrochloride; N-[4-[(3-Chloro-4-fluorophenyl)amino]-7-[3-(4-morpholinyl)propoxy]-6-quinazolinyl]-2-propenamide dihydrochloride; N-[4-(3-chloro-4-fluoroanilino)-7-(3-morpholin-4-ylpropoxy)quinazolin-6-yl]prop-2-enamide; N-[4-(3-chloro-4-fluoroanilino)-7-(3-morpholin-4-ylpropoxy)quinazolin-6-yl]prop-2-enamide dihydrochloride; N-(4-(3-chloro-4-fluorophenyl)amino)-7-(3-morpholin-4-yl)propoxy)quinazolin-6-yl)prop-2-enamide dihydrochloride; N-{4-[(3-chloro-4-fluorophenyl)amino]-7-[3-(morpholin-4-yl)propoxy]quinazolin-6-yl}prop-2-enamide; N-(4-((3-Chloro-4-fluorophenyl)amino)-7-(3-(morpholin-4-yl)propoxy)quinazolin-6-yl)prop-2-enamide; 2-Propenamide, N-(4-((3-chloro-4-fluorophenyl) amino)-7-(3-(4-morpholinyl) propoxy)-6-quinazolinyl)-, dihydrochloride; 2-Propenamide, N-(4-((3-chloro-4-fluorophenyl)amino)-7-(3-(4-morpholinyl)propoxy)-6-quinazolinyl)-, dihydrochloride
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Triciribine prodrug | Phase 1/2 | [5] | ||
| Synonyms |
TSR-826; Triciribine prodrug (oral, cancer); Triciribine prodrug (oral, cancer), TSRL
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BMS-754807 | Phase 1 | [10] | ||
| Synonyms |
1001350-96-4; BMS 754807; BMS754807; UNII-W9E3353E8J; CHEMBL575448; CHEBI:88339; W9E3353E8J; 1-{4-[(3-cyclopropyl-1H-pyrazol-5-yl)amino]pyrrolo[2,1-f][1,2,4]triazin-2-yl}-N-(6-fluoropyridin-3-yl)-2-methyl-L-prolinamide; W-204348; J-501009; 2-Pyrrolidinecarboxamide, 1-[4-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]pyrrolo[2,1-f][1,2,4]triazin-2-yl]-N-(6-fluoro-3-pyridinyl)-2-methyl-, (2S)-;2-Pyrrolidinecarboxamide, 1-[4-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]pyrrolo[2,1-f][1,2,4]triazin-2-yl]-N-(6-fluoro-3-pyridin
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ARQ 751 | Phase 1 | [4] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| M2698 | Phase 1 | [4] | ||
| Synonyms |
HXAUJHZZPCBFPN-QGZVFWFLSA-N; 1379545-95-5; SCHEMBL15262358; EX-A1187; AKOS030627134; M2698(MSC-2363318A)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28460551-Compound-6 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Squalestatin 1 | Terminated | [12] | ||
| Synonyms |
Zaragozic acid A; Squalestatin; 142561-96-4; ZARAGOZIC ACIDS A; UNII-1117HVX02L; CHEMBL280978; CHEBI:75170; 1117HVX02L; 1S-((4S-acetoxy-5R-methyl-3-methylene-6-phenylhexyl)-6-(E)-4S,6S-dimethyloct-2-enoyloxy)-4,7S-dihydroxy-2,8-dioxabicyclo[321]octane-3S,4S,5R-tricarboxylic acid; L-erythro-L-glycero-D-altro-7-Trideculo-7,4-furanosonic acid, 2,7-anhydro-3,4-di-C-carboxy-8,9,10,12,13-pentadeoxy-10-methylene-12-(phenylmethyl)-, 11-acetate 5-(4,6-dimethyl-2-octenoate), (5(2E,4S,6S),7S)-; Squalestatin 1, Glaxo; Zaragozic acid A, Glaxo
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MYRIOCIN | Investigative | [12] | ||
| Synonyms |
thermozymocidin; 35891-70-4; ISP-I; ISP-1; UNII-YRM4E8R9ST; (2S,3R,4R,6E)-2-Amino-3,4-dihydroxy-2-(hydroxymethyl)-14-oxo-6-eicosenoic acid; YRM4E8R9ST; Myriocin, Mycelia sterilia; Myriocin from Mycelia sterilia; CHEBI:582124; NCGC00163597-02; NCGC00163597-03; DSSTox_CID_26360; DSSTox_RID_81561; (2S,3R,4R,6E)-2-amino-3,4-dihydroxy-2-(hydroxymethyl)-14-oxoicos-6-enoic acid; DSSTox_GSID_46360; [2S,3R,4R]-(E)-2-Amino-3,4-dihydroxy-2-[hydroxymethyl]-14-oxo-6-eicosenoic Acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| LD-101 | Investigative | [5] | ||
| Synonyms |
AKT-SI-1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| A-674563 | Investigative | [13] | ||
| Synonyms |
A 674563; A674563
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 11 nM | |||
| External Link | ||||
| Lactoquinomycin | Investigative | [14] | ||
| Synonyms |
SCHEMBL12324296
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 149 nM | |||
| External Link | ||||
| VLI-27 | Investigative | [5] | ||
| Synonyms |
AKT inhibitor (pancreatic cancer), NovaLead Pharma
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| A-443654 | Investigative | [15] | ||
| Synonyms |
A-4436554
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 0.16 nM | |||
| External Link | ||||
| NU-1001-41 | Investigative | [5] | ||
| Synonyms |
Anti-phospho-AKT monoclonal antibodies (cancer), Nuclea Biotechnologies
Click to Show/Hide
|
|||
| External Link | ||||
| 4,5,6-trihydroxy-3-methylphthalide | Investigative | [16] | ||
| Synonyms |
CHEMBL486813; AGUVVAYMPQDJDX-UHFFFAOYSA-; BDBM50242174; 3-methyl-4,5,6-trihydroxy-phthalide; 4,5,6-Trihydroxy-3-methylisobenzofuran-1(3H)-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 19700 nM | |||
| External Link | ||||
| ALM-301 | Investigative | [5] | ||
| Synonyms |
Akt inhibitors (cancer), Almac
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BX-517 | Investigative | [17] | ||
| Synonyms |
BX517; 850717-64-5; UNII-SYV8VN8W5K; SYV8VN8W5K; pdk-1 inhibitors; BX517(PDK1 inhibitor2); Indolinone based inhibitor, 4i; SCHEMBL5567818; CHEMBL228654; 5-Ureido-3-(1-(pyrrol-2-yl)ethylidene)indolin-2-one; BDBM17004; MolPort-046-033-615; BCP16225; EX-A2243; ZINC14962724; AKOS032945106; CS-6066; Urea, N-(2,3-dihydro-2-oxo-3-((3Z)-1-(1H-pyrrol-2-yl)ethylidene)-1H-indol-5-yl)-; Urea, N-(2,3-dihydro-2-oxo-3-(1-(1H-pyrrol-2-yl)ethylidene)-1H-indol-5-yl)-; Urea, (2,3-dihydro-2-oxo-3-(1-(1H-pyrrol-2-yl)ethylidene)-1H-indol-5-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Inositol 1,3,4,5-Tetrakisphosphate | Investigative | [18] | ||
| Synonyms |
Inositol-1,3,4,5-tetraphosphate; Ins-1,3,4,5-P4; 1D-myo-inositol 1,3,4,5-tetrakisphosphate; Inositol-1,3,4,5-tetrakisphosphate; inositol-(1,3,4,5)-tetrakisphosphate; Inositol 1,3,4,5-tetraphosphate; myo-Inositol-1,3,4,5-tetrakisphosphate; CHEMBL23552; D-myo-inositol 1,3,4,5-tetrakisphosphate; CHEBI:16783; myo-Inositol, 1,3,4,5-tetrakis(dihydrogen phosphate); 1D-myo-inositol 1,3,4,5-tetrakis(dihydrogen phosphate); Ins(1,3,4,5)P4; 1bwn; 4IP; 102850-29-3; myo-Inositol 1,3,4,5-tetraphosphate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (Z)-3-((1H-pyrrol-2-yl)methylene)indolin-2-one | Investigative | [17] | ||
| Synonyms |
oxindole i; CHEMBL86755; 3-(1H-Pyrrol-2-ylmethylene)-1,3-dihydroindol-2-one; oxindole 1; AC1NZGXV; K00027; Indolinone based inhibitor, 1; SCHEMBL1162655; SCHEMBL13819612; BDBM17015; MolPort-023-197-743; SEZFNTZQMWJIAI-FLIBITNWSA-N; ZINC3874586; HSCI1_000049; NCGC00343760-01; BRD-K51816706-001-01-7; (3Z)-3-(1H-pyrrol-2-ylmethylidene)-1H-indol-2-one; 3-[(1H-Pyrrole-2-yl)methylene]-1H-indole-2(3H)-one; Z-(1H-Pyrrol-2-ylmethylene)-1,3-dihydro-indol-2-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2300 nM | |||
| External Link | ||||
| Akt inhibitor VIII | Investigative | [19] | ||
| Synonyms |
isozyme-selective, Akti-1/2
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 58 nM | |||
| External Link | ||||
| SB-747651A | Investigative | [20] | ||
| Synonyms |
CHEMBL188434; compound 26; SCHEMBL4719834; GTPL8130; BDBM24996; oxadiazole-containing compound, 9; MBCJUIJWPYUEBX-UHFFFAOYSA-N; ZINC13998530; NCGC00273984-05; NCGC00273984-03; SB-747651; 4-{1-ethyl-7-[(piperidin-4-ylamino)methyl]-1H-imidazo[4,5-c]pyridin-2-yl}-1,2,5-oxadiazol-3-amine; [2-(4-Amino-furazan-3-yl)-1-ethyl-1H-imidazo[4,5-c]pyridin-7-ylmethyl]-piperidin-4-yl-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 200 nM | |||
| External Link | ||||
| PMID20005102C1 | Investigative | [21] | ||
| Synonyms |
GTPL8181; BDBM50305878; B99
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 nM | |||
| External Link | ||||
| STAUROSPORINONE | Investigative | [22] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Ro31-8220 | Investigative | [22] | ||
| Synonyms |
Bisindolylmaleimide IX; ro 31-8220; 125314-64-9; Ro 31 8220; Ro 318220; UNII-W9A0B5E78O; Ro-318220; Ro-31-8220; CHEMBL6291; W9A0B5E78O; CHEBI:38912; 3-{3-[4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-1H-indol-1-yl}propyl carbamimidothioate; 3-{3-[4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-1H-indol-1-yl}propyl imidothiocarbamate; CHEMBL1591531; Carbamimidothioic acid, 3-(3-(2,5-dihydro-4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-1H-pyrrol-3-yl)-1H-indol-1-yl)propyl; bisindolymaleimide IX
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BMS-536924 | Investigative | [23] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| KN-62 | Investigative | [22] | ||
| Synonyms |
KN-62 (non-isomeric); GTPL6001; HMS3229A04; CCG-206863
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CI-1040 | Investigative | [22] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 4,5,6,7-tetrabromo-1H-benzo[d][1,2,3]triazole | Investigative | [24] | ||
| Synonyms |
4,5,6,7-tetrabromobenzotriazole
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-[(3,5-diamino-1H-pyrazol-4-yl)diazenyl]phenol | Investigative | [25] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Bisindolylmaleimide-I | Investigative | [22] | ||
| Synonyms |
Bisindolylmaleimide i; 133052-90-1; GF 109203X; GF109203X; Go 6850; GF-109203X; RBT205 INHIBITOR; Go-6850; UNII-L79H6N0V6C; Bisindolylmaleimide I (GF 109203X); CHEMBL7463; 3-{1-[3-(DIMETHYLAMINO)PROPYL]-1H-INDOL-3-YL}-4-(1H-INDOL-3-YL)-1H-PYRROLE-2,5-DIONE; 3-(1-(3-(Dimethylamino)propyl)-1H-indol-3-yl)-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione; L79H6N0V6C; QMGUOJYZJKLOLH-UHFFFAOYSA-N; 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl)maleimide; GF-109203; Go6850
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RO-316233 | Investigative | [22] | ||
| Synonyms |
119139-23-0; bisindolylmaleimide iv; 3,4-di(1H-indol-3-yl)-1H-pyrrole-2,5-dione; Arcyriarubin A; 3,4-Bis(3-indolyl)maleimide; 3,4-Di-1H-indol-3-yl-1H-pyrrole-2,5-dione; UNII-MBK3OO5K8T; BIM IV; 3,4-bis(1H-indol-3-yl)pyrrole-2,5-dione; MBK3OO5K8T; CHEMBL266487; 3,4-bis(1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione; DQYBRTASHMYDJG-UHFFFAOYSA-N; 2,3-bis(1H-Indol-3-yl)maleimide; 1H-Pyrrole-2,5-dione, 3,4-di-1H-indol-3-yl-; Ro-31-6233; AK-15401; 3,4-bis(3-indolyl)-1H-pyrrole-2,5-dione; Bisindoylmaleimide; Bisindolyl deriv. 3
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B72: Gastric cancer | 81 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Leniolisib | Approved | [26] | ||
| Synonyms |
1354690-24-6; Leniolisib free base; UNII-L22772Z9CP; (S)-1-(3-((6-(6-methoxy-5-(trifluoromethyl)pyridin-3-yl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-yl)amino)pyrrolidin-1-yl)propan-1-one; L22772Z9CP; 1354690-24-6 (free base); leniolisib(CDZ 173); CDZ173; CDZ-173; 1-[(3S)-3-[[6-[6-methoxy-5-(trifluoromethyl)pyridin-3-yl]-7,8-dihydro-5H-pyrido[4,3-d]pyrimidin-4-yl]amino]pyrrolidin-1-yl]propan-1-one; Leniolisib [INN]; Leniolisib (CDZ173); Leniolisib (USAN/INN); CDZ173-NX; SCHEMBL323054; GTPL9424; CHEMBL3643413; BDBM118299; EX-A2854; MFCD30470232; s8752; ZB1510; CS-7524; DC22326; SB18839; Example 67 [WO2012004299]; 1-{(S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-y; 1-{(S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-ylamino]-pyrrolidin-1-yl}-propan-1-one; AS-56217; HY-17635; A16796; D11158; US8653092, 67; Q27282602; 1-Propanone, 1-((3S)-3-((5,6,7,8-tetrahydro-6-(6-methoxy-5-(trifluoromethyl)-3-pyridinyl)pyrido(4,3-d)pyrimidin-4-yl)amino)-1-pyrrolidinyl)-; 9NQ
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [4] | ||
| External Link | ||||
| Bavencio | Approved | [4] | ||
| External Link | ||||
| Tebentafusp | Approved | [27] | ||
| External Link | ||||
| Merimepodib | Approved | [28] | ||
| Synonyms |
Merimebodib; Merimepodib [USAN:INN]; Tyverb/Tykerb; MMPD; 198821-22-6; 2ZL2BA06FU; C23H24N4O6; CHEMBL304087; MERIMEPODIB, VI-21497, VX-497; UNII-2ZL2BA06FU; VI-21497; VX-497; VX497; Vx 497; carbamic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Taxol | Approved | [8] | ||
| Synonyms |
C47H51NO14; weekly paclitaxel; Micellar Paclitaxel; Paclitaxel [USAN:INN:BAN]; SCHEMBL15000506; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha))-
Click to Show/Hide
|
|||
| External Link | ||||
| Ramucirumab | Approved | [29] | ||
| Synonyms |
LY3009806
Click to Show/Hide
|
|||
| External Link | ||||
| Tucatinib | Approved | [30] | ||
| Synonyms |
Irbinitinib; 937263-43-9; ONT-380; UNII-234248D0HH; 234248D0HH; N6-(4,4-Dimethyl-4,5-dihydrooxazol-2-yl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)quinazoline-4,6-diamine; 4,6-Quinazolinediamine, N6-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)-; ONT 380; 4,6-QuinazolinediaMine, N6-(4,5-dihydro-4,4-diMethyl-2-oxazolyl)-N4-[3-Methyl-4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)phenyl]-; Tucatinib [USAN:INN]; 6-DIAMINE
Click to Show/Hide
|
|||
| External Link | ||||
| Antacids | Approved | [31] | ||
| External Link | ||||
| Trastuzumab | Approved | [4] | ||
| Synonyms |
Herceptin; Herceptin (TN); Trastuzumab (INN); Trastuzumab (genetical recombination); Trastuzumab (genetical recombination) (JAN); Trastuzumab (ERBB2 mAb inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Carbamazepine | Phase 3 | [32] | ||
| Synonyms |
Carbamazepine (iv, epilepsy); Carbamazepine (iv, epilepsy), Lundbeck; Carbamazepine (iv, epilepsy), Ovation Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| Margetuximab | Approved | [4] | ||
| External Link | ||||
| Nivolumab | Approved | [4] | ||
| External Link | ||||
| GRANITE | Phase 3 | [33] | ||
| Synonyms |
Penoxsulam; 219714-96-2; 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; UNII-784ELC1SCZ; 784ELC1SCZ; CHEBI:81776; 2-(2,2-difluoroethoxy)-n-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; Penoxsulam [ISO]; PXD; DSSTox_CID_14803; DSSTox_RID_79204; DSSTox_GSID_34803; SCHEMBL116968; CHEMBL1895913; DTXSID0034803; HSDB 7887; AMY12535; BCP18718; EBD18529; Tox21_301010; MFCD07363876; ZINC13827750; AKOS025401685; NCGC00163715-01; NCGC00163715-02; NCGC00163715-03; NCGC00254912-01; AC-24494; Penoxsulam 100 microg/mL in Acetonitrile; CAS-219714-96-2; FT-0696708; Penoxsulam, PESTANAL(R), analytical standard; C18481; Q22808507; 2-(2,2-Difluoroethoxy)-6-trifluoromethyl-N-(5, 8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)benzenesulfonamide; 2-(2,2-Difluoroethoxy)-N-(5,8-dimethoxy[1,2,4]-triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; 2-(2,2-difluoroethoxy)-N-{5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl}-6-(trifluoromethyl)benzene-1-sulfonamide; 2-(2,2-difluoroethyl)-N-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; Benzenesulfonamide, 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy(1,2,4)triazolo(1,5-c)pyrimidin-2-yl)-6-(trifluoromethyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Zolbetuximab | Phase 3 | [34] | ||
| Synonyms |
IMAB362
Click to Show/Hide
|
|||
| External Link | ||||
| Tusamitamab ravtansine | Phase 3 | [35] | ||
| Synonyms |
SAR408701
Click to Show/Hide
|
|||
| External Link | ||||
| Andecaliximab | Phase 3 | [36] | ||
| External Link | ||||
| ABP 980 | Phase 3 | [37] | ||
| External Link | ||||
| GS-5745 | Phase 3 | [8] | ||
| External Link | ||||
| S-1 | Phase 3 | [38] | ||
| Synonyms |
Ciprofibrate-coa; Ciprofibrate-coenzyme A; Coenzyme A, ciprofibrate-; AC1L4TRG; AC1Q3T4H; 111900-25-5; s-{1-[(2r,3s,4r,5r)-5-(6-amino-9h-purin-9-yl)-4-hydroxy-3-(phosphonooxy)tetrahydrofuran-2-yl]-3,5,9-trihydroxy-8,8-dimethyl-3,5-dioxido-10,14-dioxo-2,4,6-trioxa-11,15-diaza-3; E5,5; E5-diphosphaheptadecan-17-yl} 2-[4-(2,2-dichlorocyclopropyl)phenoxy]-2-methylpropanethioate(non-preferred name); Coenzyme A, S-(2-(4-(2,2-dichlorocyclopropyl)phenoxy)-2-methylpropanoate)
Click to Show/Hide
|
|||
| External Link | ||||
| Lonsurf | Phase 3 | [4] | ||
| External Link | ||||
| GDC-0068 | Phase 3 | [8] | ||
| Synonyms |
RG7440
Click to Show/Hide
|
|||
| External Link | ||||
| Edotecarin | Phase 3 | [39] | ||
| Synonyms |
ED-749; Edotecarin < Prop INN; J-107088; PF-804950; 12-(beta-D-Glucopyranosyl)-2,10-dihydroxy-6-[2-hydroxy-1-(hydroxymethyl)ethylamino]-6,7,12,13-tetrahydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-5,7-dione
Click to Show/Hide
|
|||
| External Link | ||||
| RG3638 | Phase 3 | [40] | ||
| Synonyms |
Onartuzumab
Click to Show/Hide
|
|||
| External Link | ||||
| G17DT | Phase 3 | [41] | ||
| Synonyms |
Gastrimmune; Insegia
Click to Show/Hide
|
|||
| External Link | ||||
| DE-766 | Phase 3 | [42] | ||
| External Link | ||||
| Tesetaxel | Phase 2 | [43] | ||
| Synonyms |
DJ-927; 333754-36-2; UNII-UG97LO5M8Y; UG97LO5M8Y; Tesetaxel [INN]; DJ927; DJ 927; CHEMBL2107787; SCHEMBL12060837; DB12019; Z-3104; (2AS,2BR,3S,4S,6S,8AR,10R,11AS,11BR,13AR)-2A-ACETOXY-6-(((2R,3S)-3-((TERT-BUTOXYCARBONYL)AMINO)-3-(3-FLUOROPYRIDIN-2-YL)-2-HYDROXYPROPANOYL)OXY)-10-((DIMETHYLAMINO)METHYL)-4-HYDROXY-7,11B,14,14-TETRAMETHYL-2A,2B,3,4,5,6,8A,11A,11B,12,13,13A-DODECAHYDRO-2H-4,8-METHANOOXETO[3'',2'':3',4']BENZO[1',2':3,4]CYCLODECA[1,2-D][1,3]DIOXOL-3-YL BENZOATE
Click to Show/Hide
|
|||
| External Link | ||||
| Nelipepimut S | Phase 3 | [44] | ||
| Synonyms |
E75
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-986205 | Phase 3 | [4] | ||
| Synonyms |
KRTIYQIPSAGSBP-KLAILNCOSA-N; 1923833-60-6; BMS986205; UNII-0A7729F42K; 0A7729F42K; GTPL9707; SCHEMBL18826792; SCHEMBL17740982; SCHEMBL19105151; EX-A2606; AKOS032954040; HY-101560; CS-0021719; Q29213697; (R)-N-(4-chlorophenyl)-2-((1s,4S)-4-(6-fluoroquinolin-4-yl)cyclohexyl)propanamide; (2R)-N-(4-chlorophenyl)-2-[4-(6-fluoroquinolin-4-yl)cyclohexyl]propanamide; (2R)-N-(4-Chlorophenyl)-2-(4-(6-fluoro-4-quinolyl)cyclohexyl)propanamide, cis; Cyclohexaneacetamide, N-(4-chlorophenyl)-4-(6-fluoro-4-quinolinyl)-alpha-methyl-, cis-(alphaR)-
Click to Show/Hide
|
|||
| External Link | ||||
| Rivoceranib | Phase 3 | [4] | ||
| External Link | ||||
| Claudiximab | Phase 3 | [4] | ||
| Synonyms |
IMAB-362; Anti-GC182 mAbs (cancer), Ganymed; Anti-GC182 monoclonal antibodies (cancer), Ganymed; Anti-CLDN18-2 mAbs (cancer), Ganymed; Anti-CLDN18-2 monoclonal antibodies (cancer), Ganymed
Click to Show/Hide
|
|||
| External Link | ||||
| OS-440 | Phase 3 | [45] | ||
| Synonyms |
CNS modulator (spasticity), Osmotica
Click to Show/Hide
|
|||
| External Link | ||||
| Oraxol | Phase 3 | [4] | ||
| External Link | ||||
| ICI 118,551 | Phase 3 | [8] | ||
| Synonyms |
Ici 118551; (2R,3S)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(propan-2-ylamino)butan-2-ol; CHEMBL198059; CHEBI:73289; ICI-118551; ICI118551; erythro-DL-1-(7-Methylindan-4-yloxy)-3-isopropylaminobutan-2-ol; (2R,3S)-3-(isopropylamino)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]butan-2-ol; 2-Butanol, 1-((2,3-dihydro-7-methyl-1H-inden-4-yl)oxy)-3-((1-methylethyl)amino)-, (2R,3S)-rel-; 2-Butanol, 1-((2,3-dihydro-7-methyl-1H-inden-4-yl)oxy)-3-((1-methylethyl)amino)-, (R*,S*)-(+-)-; ICI-118,551; Ici 111,581; AC1NUNSO
Click to Show/Hide
|
|||
| External Link | ||||
| Evorpacept | Phase 2/3 | [46] | ||
| Synonyms |
ALX148
Click to Show/Hide
|
|||
| External Link | ||||
| BNT141 | Phase 2 | [47] | ||
| External Link | ||||
| Anti-LAG3 | Phase 2 | [37] | ||
| External Link | ||||
| GSK1292263 | Phase 2 | [48] | ||
| External Link | ||||
| MM-111 | Phase 2 | [49] | ||
| External Link | ||||
| Plevitrexed | Phase 2 | [50] | ||
| Synonyms |
ZD 9331; ZD9331; 153537-73-6; Plevitrexed [INN]; ZD-9331; NSC 696259; UNII-L9P2881C3H; CHEMBL126648; (2s)-2-[(2-fluoro-4-{[(4-hydroxy-2,7-dimethylquinazolin-6-yl)methyl](prop-2-yn-1-yl)amino}benzoyl)amino]-4-(2h-tetrazol-5-yl)butanoic acid; L9P2881C3H; Plevitrexed (INN); 172521-94-7; (2S)-2-[[4-[(2,7-dimethyl-4-oxo-1H-quinazolin-6-yl)methyl-prop-2-ynylamino]-2-fluorobenzoyl]amino]-4-(2H-tetrazol-5-yl)butanoic acid; 1H-Tetrazole-5-butanoic acid,
Click to Show/Hide
|
|||
| External Link | ||||
| DS-8201 | Phase 1 | [37] | ||
| Synonyms |
9-Aminofluorene; 9H-Fluoren-9-amine; 525-03-1; FLUOREN-9-AMINE; Fluoren-9-ylamine; UNII-4NHO2K4K5B; CCRIS 7000; BRN 2209545; 4NHO2K4K5B; OUGMRQJTULXVDC-UHFFFAOYSA-N; fluorene-9-ylamine; 9-Amino-fluoren; 9-amino-fluorene; 9H-9-fluorenamine; 9H-fluoren-9-yl-amine; AC1L1VP5; 4-12-00-03390 (Beilstein Handbook Reference); SCHEMBL353865; AC1Q53A2; AC1Q53A1; KS-00000JGC; CTK1H0380; DTXSID90200496; MolPort-001-794-448; HMS1780P20; 9H-fluoren-9-ylamine hydrochloride; ZINC1724407; ALBB-023296; CA-733; SBB005783; AKOS000264388; MCULE-8757055914; DS-
Click to Show/Hide
|
|||
| External Link | ||||
| XL880 | Phase 2 | [51] | ||
| Synonyms |
GSK 089; GSK 1363089; GSK1363089; XL 880; GSK1363089, GSK089, foretinib, EXEL-2880, XL880; 88Z; MET inhibitors
Click to Show/Hide
|
|||
| External Link | ||||
| Matuzumab | Phase 2 | [52] | ||
| Synonyms |
EMD-62000; EMD-72000; Anti-EGF receptor humanized antibody (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals; Anti-EGFR humanized mAb (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals; Anti-epidermal growth factor receptor humanized antibody (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| BAY-57-9352 | Phase 2 | [8] | ||
| Synonyms |
Telatinib; Bay 57-9352
Click to Show/Hide
|
|||
| External Link | ||||
| Bemarituzumab | Phase 2 | [53] | ||
| External Link | ||||
| PEGPH20 | Phase 2 | [4] | ||
| External Link | ||||
| Plevitrexed (R)-isomer | Phase 2 | [54] | ||
| Synonyms |
YW3548
Click to Show/Hide
|
|||
| External Link | ||||
| APR-246 | Phase 2 | [55] | ||
| Synonyms |
Eprenetapopt
Click to Show/Hide
|
|||
| External Link | ||||
| CRS-207 | Phase 2 | [44] | ||
| External Link | ||||
| Opdivo + Yervoy | Phase 3 | [4] | ||
| External Link | ||||
| CT-041 | Phase 1/2 | [56] | ||
| External Link | ||||
| BPX-601 | Phase 1/2 | [57] | ||
| External Link | ||||
| Anti-MUC1 CAR-T cells | Phase 1/2 | [58] | ||
| External Link | ||||
| Anti-Mesothelin CAR-T cells | Phase 1/2 | [59] | ||
| External Link | ||||
| Anti-HER2 CAR-T | Phase 1/2 | [60] | ||
| External Link | ||||
| CAR-T Cells targeting EpCAM | Phase 1/2 | [61] | ||
| External Link | ||||
| PAT-SC1 | Phase 1/2 | [62] | ||
| Synonyms |
SC-1; Adjuvant therapy (gastric cancer), University of Wurzburg; SC-1 (gastric cancer), CAT; SC-1 (gastric cancer), Debiopharm; SC-1 (gastric cancer), Patrys; SC-1 (stomach cancer), OncoMab
Click to Show/Hide
|
|||
| External Link | ||||
| ASP2138 | Phase 1 | [63] | ||
| External Link | ||||
| SAR443216 | Phase 1 | [64] | ||
| External Link | ||||
| AMG 199 | Phase 1 | [65] | ||
| External Link | ||||
| AMG 910 | Phase 1 | [66] | ||
| External Link | ||||
| Alofanib | Phase 1 | [67] | ||
| Synonyms |
1612888-66-0; 3-(N-(4-methyl-2-nitro-5-(pyridin-3-yl)phenyl)sulfamoyl)benzoic acid; RPT-835(alofanib); UNII-LQX7RFK8MZ; RPT-835; RPT835; LQX7RFK8MZ; ES000835; Alofanib [INN]; Alofanib(RPT835); Syn007154; CHEMBL4594436; SCHEMBL18660613; AMY16650; BCP31905; EX-A2731; MFCD30533418; NSC790182; s8754; Benzoic acid, 3-(((4-methyl-2-nitro-5-(3-pyridinyl)phenyl)amino)sulfonyl)-; NSC-790182; SB19665; AC-31695; AK668992; AS-56846; HY-17601; CS-0014684; RPT 835; Q27283135; 3-{[4-methyl-2-nitro-5-(pyridin-3-yl)phenyl]sulfamoyl}benzoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| HER2-specific CAR T cell | Phase 1 | [68] | ||
| External Link | ||||
| Anti-CEA-CAR T | Phase 1 | [69] | ||
| External Link | ||||
| XR-5944 | Phase 1 | [70] | ||
| Synonyms |
MLN-944; XR-11576 analogs; XR-5000 analogs; XR-5942
Click to Show/Hide
|
|||
| External Link | ||||
| A168 | Phase 1 | [71] | ||
| External Link | ||||
| EGFR806-specific CAR T cell | Phase 1 | [72] | ||
| External Link | ||||
| AbGn-107 | Phase 1 | [4] | ||
| External Link | ||||
| FPA144 | Phase 1 | [37] | ||
| External Link | ||||
| Minnelide 001 | Phase 1 | [8] | ||
| External Link | ||||
| CAR-T cells targeting EpCAM | Phase 1 | [73] | ||
| External Link | ||||
| Anti-CEA CAR-T cells | Phase 1 | [74] | ||
| External Link | ||||
| EPCAM-targeted CAR-T cells | Clinical trial | [75] | ||
| External Link | ||||
| PMID28460551-Compound-1 | Patented | [11] | ||
| External Link | ||||
| Conjugated 3-(indolyl)-and 3-(azaindolyl)-4-arylmaleimide compound 1 | Patented | [76] | ||
| Synonyms |
PMID28621580-Compound-WO2012084683c62
Click to Show/Hide
|
|||
| External Link | ||||
| TOPIXANTRONE HYDROCHLORIDE | Discontinued in Phase 2 | [77] | ||
| Synonyms |
SCHEMBL1418986; Topixantrone hydrochloride < Prop INNM; BBR-3409 (dimaleate); 5-[2-(Dimethylamino)ethylamino]-2-[2-(2-hydroxyethylamino)ethyl]indazolo[4,3-gh]isoquinolin-6(2H)-one dihydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| MDL 101,731 | Discontinued in Phase 2 | [78] | ||
| Synonyms |
Tezacitabine; Fmdc cpd; 130306-02-4; UNII-7607Y95N9S; Mdl 101731; (E)-2'-Deoxy-2'-(fluoromethylene) cytidine; MDL-101731; 2'-Deoxy-2'-(fluoromethylene)cytidine; 7607Y95N9S; Cytidine, 2'-deoxy-2'-(fluoromethylene)-, (2E)-; (E)-2'-Deoxy-2'-(fluoromethylene)cytidine; Tezacitabine [INN]; tezaciabine; Tezacitabine, anhydrous; AC1O5KIG; SCHEMBL18724; SCHEMBL18725; Tezacitabine, anhydrous [INN]; CHEMBL2105467; C10H12FN3O4; DTXSID10156446; GFFXZLZWLOBBLO-ASKVSEFXSA-N; ZINC3777826; KW-2331
Click to Show/Hide
|
|||
| External Link | ||||
| BBR-3438 | Discontinued in Phase 2 | [79] | ||
| Synonyms |
Nortopixantrone; UNII-PH2639TAB4; PH2639TAB4; Nortopixantrone [INN:BAN]; AC1MI4ZO; CHEMBL150303; SCHEMBL7804438
Click to Show/Hide
|
|||
| External Link | ||||
| IPI-493 | Discontinued in Phase 1 | [80] | ||
| Synonyms |
[(3R,5R,6S,7R,8E,10R,11R,12Z,14E)-21-amino-6-hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate; AC1NS08X; SCHEMBL16226496; SCHEMBL16225851
Click to Show/Hide
|
|||
| External Link | ||||
| Kanjinti | Application submitted | [4] | ||
| External Link | ||||
| Anti-CD9 mab | Investigative | [81] | ||
| Synonyms |
ALB-6; Anti-CD9 mAb (gastric cancer); Anti-CD9 mAb (gastric cancer), Osaka University
Click to Show/Hide
|
|||
| External Link | ||||
References