m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03498
|
[1], [2] | |||
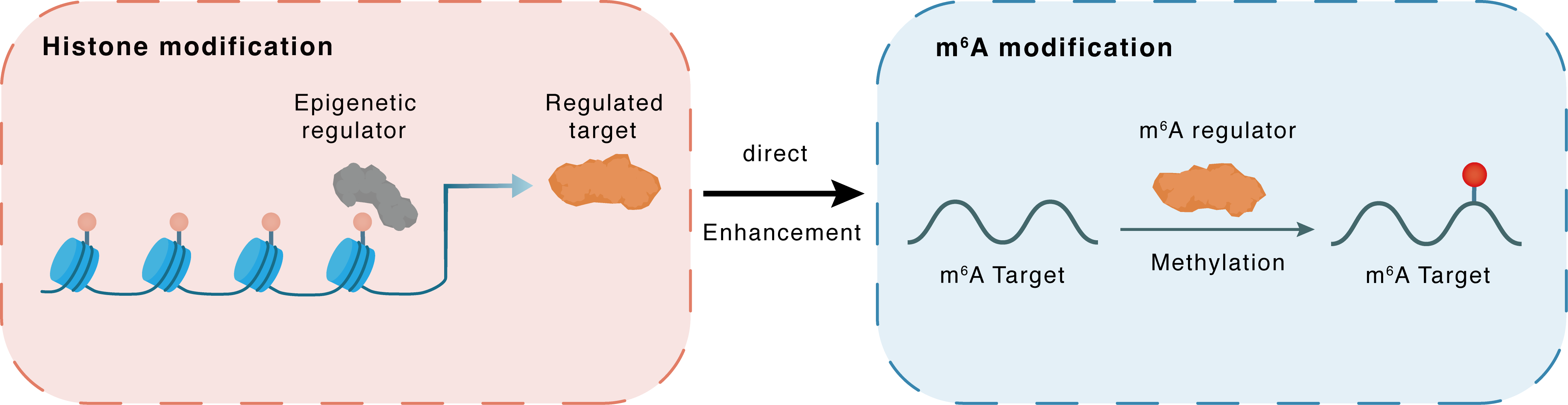 Histone modification
H3K18la
Epigenetic Regulator
METTL14
Direct
Enhancement
m6A modification
PIK3CA
PIK3CA
METTL14
Methylation
Histone modification
H3K18la
Epigenetic Regulator
METTL14
Direct
Enhancement
m6A modification
PIK3CA
PIK3CA
METTL14
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 14 (METTL14) | WRITER | |||
| m6A Target | PI3-kinase subunit alpha (PI3k/PIK3CA) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Regulated Target | Histone H3 lysine 18 lactylation (H3K18la) | View Details | |||
| Downstream Gene | METTL14 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | Histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | Histone H3 lysine 18 lactylation (H3K18la) was found to upregulate METTL14 expression. In conclusion, METTL14 knockdown promotes stemness in GC by mediating m6A modification of ATF5 mRNA, which activates the WDR74/beta-catenin axis, making METTL14 a potential therapeutic target for gastric cancer treatment. The m6A modification level was decreased in GC and METTL14 was a key regulator resulting in m6A disorder in GC. METTL14 overexpression suppressed GC cell proliferation and aggression by deactivating the PI3-kinase subunit alpha (PI3k/PIK3CA)/AKT/mTOR pathway and the EMT pathway, respectively. | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
In-vitro Model |
GES-1 | Normal | Homo sapiens | CVCL_EQ22 | |
| SGC-7901 | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| MGC-803 | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| PI3-kinase subunit alpha (PI3k/PIK3CA) | 12 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Alpelisib | Approved | [3] | ||
| Synonyms |
1217486-61-7; BYL-719; BYL719; UNII-08W5N2C97Q; BYL 719; Alpelisib (BYL719); (S)-N1-(4-Methyl-5-(2-(1,1,1-trifluoro-2-methylpropan-2-yl)pyridin-4-yl)thiazol-2-yl)pyrrolidine-1,2-dicarboxamide; NVP-BYL719; (2S)-N1-[4-Methyl-5-[2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridinyl]-2-thiazolyl]-1,2-pyrrolidinedicarboxamide; CHEMBL2396661; 08W5N2C97Q; AK146107; C19H22F3N5O2S; (S)-N1-(4-Methyl-5-(2-(1,1,1-trifluoro-2-methylpropan-2-yl)-pyridin-4-yl)thiazol-2-yl)pyrrolidine-1,2-dicarboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| BAY 80-6946 | Approved | [4] | ||
| Synonyms |
Copanlisib; BAY-80-6946; Aliqopa; UNII-WI6V529FZ9; BAY80-6946; WI6V529FZ9; AK172384; BAY 80-6946 (Copanlisib); Copanlisib [USAN:INN]; Copanlisib (USAN/INN); GTPL7875; SCHEMBL1655478; SCHEMBL13084037; Copanlisib (BAY 80-6946); DTXS
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.5 nM | |||
| External Link | ||||
| GDC-0032 | Phase 2/3 | [5] | ||
| Synonyms |
Taselisib; 1282512-48-4; GDC 0032; UNII-L08J2O299M; 2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl)-1H-pyrazol-1-yl)-2-methylpropanamide; CHEMBL2387080; GDC0032; L08J2O299M; Taselisib [INN]; 2-Methyl-2-(4-{2-[3-Methyl-1-(Propan-2-Yl)-1h-1,2,4-Triazol-5-Yl]-5,6-Dihydroimidazo[1,2-D][1,4]benzoxazepin-9-Yl}-1h-Pyrazol-1-Yl)propanamide; Taselisib [USAN:INN]; GTPL7794; SCHEMBL1485247; SYN1202; DTXSID00155842; BEUQXVWXFDOSAQ-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 0.29 nM | |||
| External Link | ||||
| Buparlisib | Phase 3 | [6] | ||
| Synonyms |
BKM120
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 20 nM | |||
| External Link | ||||
| LY3023414 | Phase 2 | [7] | ||
| MOA | Modulator | |||
| External Link | ||||
| MLN1117 | Phase 1/2 | [8] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 15 nM | |||
| External Link | ||||
| PA-799 | Phase 1/2 | [9] | ||
| Synonyms |
MEN1611; CH-5033855; CH-5061565; CH-5089788; CH-5108135; CH-5111436; CH-5138134
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BLY719 | Phase 1 | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| LY3849524 | Phase 1 | [11] | ||
| Synonyms |
LOXO 783
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| HHCYH33 | Phase 1 | [12] | ||
| Synonyms |
CYH33; UNII-RCA38L4HB5; RCA38L4HB5; 1494684-28-4; Methyl (5-(6-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-morpholinopyrrolo[2,1-f][1,2,4]triazin-2-yl)-4-(trifluoromethyl)pyridin-2-yl)carbamate; CYH 33; CYH-33; SCHEMBL16622890; BDBM330295; HY-123938; CS-0087546; US9724352, I-33; Carbamic acid, N-(5-(6-((4-(methylsulfonyl)-1-piperazinyl)methyl)-4-(4-morpholinyl)pyrrolo(2,1-F)(1,2,4)triazin-2-yl)-4-(trifluoromethyl)-2-pyridinyl)-, methyl ester; Methyl N-(5-(6-((4-(methylsulfonyl)-1-piperazinyl)methyl)-4-(4-morpholinyl)pyrrolo(2,1-F)(1,2,4)triazin-2-yl)-4-(trifluoromethyl)-2-pyridinyl)carbamate; methyl N-[5-[6-[(4-methylsulfonylpiperazin-1-yl)methyl]-4-morpholin-4-ylpyrrolo[2,1-f][1,2,4]triazin-2-yl]-4-(trifluoromethyl)pyridin-2-yl]carbamate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PWT-33597 | Phase 1 | [13] | ||
| Synonyms |
PI3 kinase alpha/mTOR dual inhibitor (cancer), Pathway Therapeutics
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| ETP-46321 | Investigative | [14] | ||
| Synonyms |
1252594-99-2; CHEMBL2087474; 5-(2-((4-(methylsulfonyl)piperazin-1-yl)methyl)-8-morpholinoimidazo[1,2-a]pyrazin-6-yl)pyrimidin-2-amine; 5-[2-[(4-methylsulfonylpiperazin-1-yl)methyl]-8-morpholin-4-ylimidazo[1,2-a]pyrazin-6-yl]pyrimidin-2-amine; SCHEMBL10100315; BCP19935; ETP46321; EX-A1284; BDBM50420714; ZINC68247289; CS-3350; ETP 46321;ETP46321; HY-12340; 5-[2-[(4-methylsulfonylpiperazin-1-yl)methyl]-8-morpholin-4-yl-imidazo[1,2-a]pyrazin-6-yl]pyrimidin-2-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B72: Gastric cancer | 81 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Leniolisib | Approved | [15] | ||
| Synonyms |
1354690-24-6; Leniolisib free base; UNII-L22772Z9CP; (S)-1-(3-((6-(6-methoxy-5-(trifluoromethyl)pyridin-3-yl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-yl)amino)pyrrolidin-1-yl)propan-1-one; L22772Z9CP; 1354690-24-6 (free base); leniolisib(CDZ 173); CDZ173; CDZ-173; 1-[(3S)-3-[[6-[6-methoxy-5-(trifluoromethyl)pyridin-3-yl]-7,8-dihydro-5H-pyrido[4,3-d]pyrimidin-4-yl]amino]pyrrolidin-1-yl]propan-1-one; Leniolisib [INN]; Leniolisib (CDZ173); Leniolisib (USAN/INN); CDZ173-NX; SCHEMBL323054; GTPL9424; CHEMBL3643413; BDBM118299; EX-A2854; MFCD30470232; s8752; ZB1510; CS-7524; DC22326; SB18839; Example 67 [WO2012004299]; 1-{(S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-y; 1-{(S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-ylamino]-pyrrolidin-1-yl}-propan-1-one; AS-56217; HY-17635; A16796; D11158; US8653092, 67; Q27282602; 1-Propanone, 1-((3S)-3-((5,6,7,8-tetrahydro-6-(6-methoxy-5-(trifluoromethyl)-3-pyridinyl)pyrido(4,3-d)pyrimidin-4-yl)amino)-1-pyrrolidinyl)-; 9NQ
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [16] | ||
| External Link | ||||
| Bavencio | Approved | [16] | ||
| External Link | ||||
| Tebentafusp | Approved | [17] | ||
| External Link | ||||
| Merimepodib | Approved | [18] | ||
| Synonyms |
Merimebodib; Merimepodib [USAN:INN]; Tyverb/Tykerb; MMPD; 198821-22-6; 2ZL2BA06FU; C23H24N4O6; CHEMBL304087; MERIMEPODIB, VI-21497, VX-497; UNII-2ZL2BA06FU; VI-21497; VX-497; VX497; Vx 497; carbamic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Taxol | Approved | [19] | ||
| Synonyms |
C47H51NO14; weekly paclitaxel; Micellar Paclitaxel; Paclitaxel [USAN:INN:BAN]; SCHEMBL15000506; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha))-
Click to Show/Hide
|
|||
| External Link | ||||
| Ramucirumab | Approved | [20] | ||
| Synonyms |
LY3009806
Click to Show/Hide
|
|||
| External Link | ||||
| Tucatinib | Approved | [21] | ||
| Synonyms |
Irbinitinib; 937263-43-9; ONT-380; UNII-234248D0HH; 234248D0HH; N6-(4,4-Dimethyl-4,5-dihydrooxazol-2-yl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)quinazoline-4,6-diamine; 4,6-Quinazolinediamine, N6-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)-; ONT 380; 4,6-QuinazolinediaMine, N6-(4,5-dihydro-4,4-diMethyl-2-oxazolyl)-N4-[3-Methyl-4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)phenyl]-; Tucatinib [USAN:INN]; 6-DIAMINE
Click to Show/Hide
|
|||
| External Link | ||||
| Antacids | Approved | [22] | ||
| External Link | ||||
| Trastuzumab | Approved | [16] | ||
| Synonyms |
Herceptin; Herceptin (TN); Trastuzumab (INN); Trastuzumab (genetical recombination); Trastuzumab (genetical recombination) (JAN); Trastuzumab (ERBB2 mAb inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Carbamazepine | Phase 3 | [23] | ||
| Synonyms |
Carbamazepine (iv, epilepsy); Carbamazepine (iv, epilepsy), Lundbeck; Carbamazepine (iv, epilepsy), Ovation Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| Margetuximab | Approved | [16] | ||
| External Link | ||||
| Nivolumab | Approved | [16] | ||
| External Link | ||||
| GRANITE | Phase 3 | [24] | ||
| Synonyms |
Penoxsulam; 219714-96-2; 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; UNII-784ELC1SCZ; 784ELC1SCZ; CHEBI:81776; 2-(2,2-difluoroethoxy)-n-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; Penoxsulam [ISO]; PXD; DSSTox_CID_14803; DSSTox_RID_79204; DSSTox_GSID_34803; SCHEMBL116968; CHEMBL1895913; DTXSID0034803; HSDB 7887; AMY12535; BCP18718; EBD18529; Tox21_301010; MFCD07363876; ZINC13827750; AKOS025401685; NCGC00163715-01; NCGC00163715-02; NCGC00163715-03; NCGC00254912-01; AC-24494; Penoxsulam 100 microg/mL in Acetonitrile; CAS-219714-96-2; FT-0696708; Penoxsulam, PESTANAL(R), analytical standard; C18481; Q22808507; 2-(2,2-Difluoroethoxy)-6-trifluoromethyl-N-(5, 8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)benzenesulfonamide; 2-(2,2-Difluoroethoxy)-N-(5,8-dimethoxy[1,2,4]-triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; 2-(2,2-difluoroethoxy)-N-{5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl}-6-(trifluoromethyl)benzene-1-sulfonamide; 2-(2,2-difluoroethyl)-N-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; Benzenesulfonamide, 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy(1,2,4)triazolo(1,5-c)pyrimidin-2-yl)-6-(trifluoromethyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Zolbetuximab | Phase 3 | [25] | ||
| Synonyms |
IMAB362
Click to Show/Hide
|
|||
| External Link | ||||
| Tusamitamab ravtansine | Phase 3 | [26] | ||
| Synonyms |
SAR408701
Click to Show/Hide
|
|||
| External Link | ||||
| Andecaliximab | Phase 3 | [27] | ||
| External Link | ||||
| ABP 980 | Phase 3 | [28] | ||
| External Link | ||||
| GS-5745 | Phase 3 | [19] | ||
| External Link | ||||
| S-1 | Phase 3 | [29] | ||
| Synonyms |
Ciprofibrate-coa; Ciprofibrate-coenzyme A; Coenzyme A, ciprofibrate-; AC1L4TRG; AC1Q3T4H; 111900-25-5; s-{1-[(2r,3s,4r,5r)-5-(6-amino-9h-purin-9-yl)-4-hydroxy-3-(phosphonooxy)tetrahydrofuran-2-yl]-3,5,9-trihydroxy-8,8-dimethyl-3,5-dioxido-10,14-dioxo-2,4,6-trioxa-11,15-diaza-3; E5,5; E5-diphosphaheptadecan-17-yl} 2-[4-(2,2-dichlorocyclopropyl)phenoxy]-2-methylpropanethioate(non-preferred name); Coenzyme A, S-(2-(4-(2,2-dichlorocyclopropyl)phenoxy)-2-methylpropanoate)
Click to Show/Hide
|
|||
| External Link | ||||
| Lonsurf | Phase 3 | [16] | ||
| External Link | ||||
| GDC-0068 | Phase 3 | [19] | ||
| Synonyms |
RG7440
Click to Show/Hide
|
|||
| External Link | ||||
| Edotecarin | Phase 3 | [30] | ||
| Synonyms |
ED-749; Edotecarin < Prop INN; J-107088; PF-804950; 12-(beta-D-Glucopyranosyl)-2,10-dihydroxy-6-[2-hydroxy-1-(hydroxymethyl)ethylamino]-6,7,12,13-tetrahydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-5,7-dione
Click to Show/Hide
|
|||
| External Link | ||||
| RG3638 | Phase 3 | [31] | ||
| Synonyms |
Onartuzumab
Click to Show/Hide
|
|||
| External Link | ||||
| G17DT | Phase 3 | [32] | ||
| Synonyms |
Gastrimmune; Insegia
Click to Show/Hide
|
|||
| External Link | ||||
| DE-766 | Phase 3 | [33] | ||
| External Link | ||||
| Tesetaxel | Phase 2 | [34] | ||
| Synonyms |
DJ-927; 333754-36-2; UNII-UG97LO5M8Y; UG97LO5M8Y; Tesetaxel [INN]; DJ927; DJ 927; CHEMBL2107787; SCHEMBL12060837; DB12019; Z-3104; (2AS,2BR,3S,4S,6S,8AR,10R,11AS,11BR,13AR)-2A-ACETOXY-6-(((2R,3S)-3-((TERT-BUTOXYCARBONYL)AMINO)-3-(3-FLUOROPYRIDIN-2-YL)-2-HYDROXYPROPANOYL)OXY)-10-((DIMETHYLAMINO)METHYL)-4-HYDROXY-7,11B,14,14-TETRAMETHYL-2A,2B,3,4,5,6,8A,11A,11B,12,13,13A-DODECAHYDRO-2H-4,8-METHANOOXETO[3'',2'':3',4']BENZO[1',2':3,4]CYCLODECA[1,2-D][1,3]DIOXOL-3-YL BENZOATE
Click to Show/Hide
|
|||
| External Link | ||||
| Nelipepimut S | Phase 3 | [35] | ||
| Synonyms |
E75
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-986205 | Phase 3 | [16] | ||
| Synonyms |
KRTIYQIPSAGSBP-KLAILNCOSA-N; 1923833-60-6; BMS986205; UNII-0A7729F42K; 0A7729F42K; GTPL9707; SCHEMBL18826792; SCHEMBL17740982; SCHEMBL19105151; EX-A2606; AKOS032954040; HY-101560; CS-0021719; Q29213697; (R)-N-(4-chlorophenyl)-2-((1s,4S)-4-(6-fluoroquinolin-4-yl)cyclohexyl)propanamide; (2R)-N-(4-chlorophenyl)-2-[4-(6-fluoroquinolin-4-yl)cyclohexyl]propanamide; (2R)-N-(4-Chlorophenyl)-2-(4-(6-fluoro-4-quinolyl)cyclohexyl)propanamide, cis; Cyclohexaneacetamide, N-(4-chlorophenyl)-4-(6-fluoro-4-quinolinyl)-alpha-methyl-, cis-(alphaR)-
Click to Show/Hide
|
|||
| External Link | ||||
| Rivoceranib | Phase 3 | [16] | ||
| External Link | ||||
| Claudiximab | Phase 3 | [16] | ||
| Synonyms |
IMAB-362; Anti-GC182 mAbs (cancer), Ganymed; Anti-GC182 monoclonal antibodies (cancer), Ganymed; Anti-CLDN18-2 mAbs (cancer), Ganymed; Anti-CLDN18-2 monoclonal antibodies (cancer), Ganymed
Click to Show/Hide
|
|||
| External Link | ||||
| OS-440 | Phase 3 | [36] | ||
| Synonyms |
CNS modulator (spasticity), Osmotica
Click to Show/Hide
|
|||
| External Link | ||||
| Oraxol | Phase 3 | [16] | ||
| External Link | ||||
| ICI 118,551 | Phase 3 | [19] | ||
| Synonyms |
Ici 118551; (2R,3S)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(propan-2-ylamino)butan-2-ol; CHEMBL198059; CHEBI:73289; ICI-118551; ICI118551; erythro-DL-1-(7-Methylindan-4-yloxy)-3-isopropylaminobutan-2-ol; (2R,3S)-3-(isopropylamino)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]butan-2-ol; 2-Butanol, 1-((2,3-dihydro-7-methyl-1H-inden-4-yl)oxy)-3-((1-methylethyl)amino)-, (2R,3S)-rel-; 2-Butanol, 1-((2,3-dihydro-7-methyl-1H-inden-4-yl)oxy)-3-((1-methylethyl)amino)-, (R*,S*)-(+-)-; ICI-118,551; Ici 111,581; AC1NUNSO
Click to Show/Hide
|
|||
| External Link | ||||
| Evorpacept | Phase 2/3 | [37] | ||
| Synonyms |
ALX148
Click to Show/Hide
|
|||
| External Link | ||||
| BNT141 | Phase 2 | [38] | ||
| External Link | ||||
| Anti-LAG3 | Phase 2 | [28] | ||
| External Link | ||||
| GSK1292263 | Phase 2 | [39] | ||
| External Link | ||||
| MM-111 | Phase 2 | [40] | ||
| External Link | ||||
| Plevitrexed | Phase 2 | [41] | ||
| Synonyms |
ZD 9331; ZD9331; 153537-73-6; Plevitrexed [INN]; ZD-9331; NSC 696259; UNII-L9P2881C3H; CHEMBL126648; (2s)-2-[(2-fluoro-4-{[(4-hydroxy-2,7-dimethylquinazolin-6-yl)methyl](prop-2-yn-1-yl)amino}benzoyl)amino]-4-(2h-tetrazol-5-yl)butanoic acid; L9P2881C3H; Plevitrexed (INN); 172521-94-7; (2S)-2-[[4-[(2,7-dimethyl-4-oxo-1H-quinazolin-6-yl)methyl-prop-2-ynylamino]-2-fluorobenzoyl]amino]-4-(2H-tetrazol-5-yl)butanoic acid; 1H-Tetrazole-5-butanoic acid,
Click to Show/Hide
|
|||
| External Link | ||||
| DS-8201 | Phase 1 | [28] | ||
| Synonyms |
9-Aminofluorene; 9H-Fluoren-9-amine; 525-03-1; FLUOREN-9-AMINE; Fluoren-9-ylamine; UNII-4NHO2K4K5B; CCRIS 7000; BRN 2209545; 4NHO2K4K5B; OUGMRQJTULXVDC-UHFFFAOYSA-N; fluorene-9-ylamine; 9-Amino-fluoren; 9-amino-fluorene; 9H-9-fluorenamine; 9H-fluoren-9-yl-amine; AC1L1VP5; 4-12-00-03390 (Beilstein Handbook Reference); SCHEMBL353865; AC1Q53A2; AC1Q53A1; KS-00000JGC; CTK1H0380; DTXSID90200496; MolPort-001-794-448; HMS1780P20; 9H-fluoren-9-ylamine hydrochloride; ZINC1724407; ALBB-023296; CA-733; SBB005783; AKOS000264388; MCULE-8757055914; DS-
Click to Show/Hide
|
|||
| External Link | ||||
| XL880 | Phase 2 | [42] | ||
| Synonyms |
GSK 089; GSK 1363089; GSK1363089; XL 880; GSK1363089, GSK089, foretinib, EXEL-2880, XL880; 88Z; MET inhibitors
Click to Show/Hide
|
|||
| External Link | ||||
| Matuzumab | Phase 2 | [43] | ||
| Synonyms |
EMD-62000; EMD-72000; Anti-EGF receptor humanized antibody (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals; Anti-EGFR humanized mAb (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals; Anti-epidermal growth factor receptor humanized antibody (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| BAY-57-9352 | Phase 2 | [19] | ||
| Synonyms |
Telatinib; Bay 57-9352
Click to Show/Hide
|
|||
| External Link | ||||
| Bemarituzumab | Phase 2 | [44] | ||
| External Link | ||||
| PEGPH20 | Phase 2 | [16] | ||
| External Link | ||||
| Plevitrexed (R)-isomer | Phase 2 | [45] | ||
| Synonyms |
YW3548
Click to Show/Hide
|
|||
| External Link | ||||
| APR-246 | Phase 2 | [46] | ||
| Synonyms |
Eprenetapopt
Click to Show/Hide
|
|||
| External Link | ||||
| CRS-207 | Phase 2 | [35] | ||
| External Link | ||||
| Opdivo + Yervoy | Phase 3 | [16] | ||
| External Link | ||||
| CT-041 | Phase 1/2 | [47] | ||
| External Link | ||||
| BPX-601 | Phase 1/2 | [48] | ||
| External Link | ||||
| Anti-MUC1 CAR-T cells | Phase 1/2 | [49] | ||
| External Link | ||||
| Anti-Mesothelin CAR-T cells | Phase 1/2 | [50] | ||
| External Link | ||||
| Anti-HER2 CAR-T | Phase 1/2 | [51] | ||
| External Link | ||||
| CAR-T Cells targeting EpCAM | Phase 1/2 | [52] | ||
| External Link | ||||
| PAT-SC1 | Phase 1/2 | [53] | ||
| Synonyms |
SC-1; Adjuvant therapy (gastric cancer), University of Wurzburg; SC-1 (gastric cancer), CAT; SC-1 (gastric cancer), Debiopharm; SC-1 (gastric cancer), Patrys; SC-1 (stomach cancer), OncoMab
Click to Show/Hide
|
|||
| External Link | ||||
| ASP2138 | Phase 1 | [54] | ||
| External Link | ||||
| SAR443216 | Phase 1 | [55] | ||
| External Link | ||||
| AMG 199 | Phase 1 | [56] | ||
| External Link | ||||
| AMG 910 | Phase 1 | [57] | ||
| External Link | ||||
| Alofanib | Phase 1 | [58] | ||
| Synonyms |
1612888-66-0; 3-(N-(4-methyl-2-nitro-5-(pyridin-3-yl)phenyl)sulfamoyl)benzoic acid; RPT-835(alofanib); UNII-LQX7RFK8MZ; RPT-835; RPT835; LQX7RFK8MZ; ES000835; Alofanib [INN]; Alofanib(RPT835); Syn007154; CHEMBL4594436; SCHEMBL18660613; AMY16650; BCP31905; EX-A2731; MFCD30533418; NSC790182; s8754; Benzoic acid, 3-(((4-methyl-2-nitro-5-(3-pyridinyl)phenyl)amino)sulfonyl)-; NSC-790182; SB19665; AC-31695; AK668992; AS-56846; HY-17601; CS-0014684; RPT 835; Q27283135; 3-{[4-methyl-2-nitro-5-(pyridin-3-yl)phenyl]sulfamoyl}benzoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| HER2-specific CAR T cell | Phase 1 | [59] | ||
| External Link | ||||
| Anti-CEA-CAR T | Phase 1 | [60] | ||
| External Link | ||||
| XR-5944 | Phase 1 | [61] | ||
| Synonyms |
MLN-944; XR-11576 analogs; XR-5000 analogs; XR-5942
Click to Show/Hide
|
|||
| External Link | ||||
| A168 | Phase 1 | [62] | ||
| External Link | ||||
| EGFR806-specific CAR T cell | Phase 1 | [63] | ||
| External Link | ||||
| AbGn-107 | Phase 1 | [16] | ||
| External Link | ||||
| FPA144 | Phase 1 | [28] | ||
| External Link | ||||
| Minnelide 001 | Phase 1 | [19] | ||
| External Link | ||||
| CAR-T cells targeting EpCAM | Phase 1 | [64] | ||
| External Link | ||||
| Anti-CEA CAR-T cells | Phase 1 | [65] | ||
| External Link | ||||
| EPCAM-targeted CAR-T cells | Clinical trial | [66] | ||
| External Link | ||||
| PMID28460551-Compound-1 | Patented | [67] | ||
| External Link | ||||
| Conjugated 3-(indolyl)-and 3-(azaindolyl)-4-arylmaleimide compound 1 | Patented | [68] | ||
| Synonyms |
PMID28621580-Compound-WO2012084683c62
Click to Show/Hide
|
|||
| External Link | ||||
| TOPIXANTRONE HYDROCHLORIDE | Discontinued in Phase 2 | [69] | ||
| Synonyms |
SCHEMBL1418986; Topixantrone hydrochloride < Prop INNM; BBR-3409 (dimaleate); 5-[2-(Dimethylamino)ethylamino]-2-[2-(2-hydroxyethylamino)ethyl]indazolo[4,3-gh]isoquinolin-6(2H)-one dihydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| MDL 101,731 | Discontinued in Phase 2 | [70] | ||
| Synonyms |
Tezacitabine; Fmdc cpd; 130306-02-4; UNII-7607Y95N9S; Mdl 101731; (E)-2'-Deoxy-2'-(fluoromethylene) cytidine; MDL-101731; 2'-Deoxy-2'-(fluoromethylene)cytidine; 7607Y95N9S; Cytidine, 2'-deoxy-2'-(fluoromethylene)-, (2E)-; (E)-2'-Deoxy-2'-(fluoromethylene)cytidine; Tezacitabine [INN]; tezaciabine; Tezacitabine, anhydrous; AC1O5KIG; SCHEMBL18724; SCHEMBL18725; Tezacitabine, anhydrous [INN]; CHEMBL2105467; C10H12FN3O4; DTXSID10156446; GFFXZLZWLOBBLO-ASKVSEFXSA-N; ZINC3777826; KW-2331
Click to Show/Hide
|
|||
| External Link | ||||
| BBR-3438 | Discontinued in Phase 2 | [71] | ||
| Synonyms |
Nortopixantrone; UNII-PH2639TAB4; PH2639TAB4; Nortopixantrone [INN:BAN]; AC1MI4ZO; CHEMBL150303; SCHEMBL7804438
Click to Show/Hide
|
|||
| External Link | ||||
| IPI-493 | Discontinued in Phase 1 | [72] | ||
| Synonyms |
[(3R,5R,6S,7R,8E,10R,11R,12Z,14E)-21-amino-6-hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate; AC1NS08X; SCHEMBL16226496; SCHEMBL16225851
Click to Show/Hide
|
|||
| External Link | ||||
| Kanjinti | Application submitted | [16] | ||
| External Link | ||||
| Anti-CD9 mab | Investigative | [73] | ||
| Synonyms |
ALB-6; Anti-CD9 mAb (gastric cancer); Anti-CD9 mAb (gastric cancer), Osaka University
Click to Show/Hide
|
|||
| External Link | ||||
References