m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03407
|
[1], [2] | |||
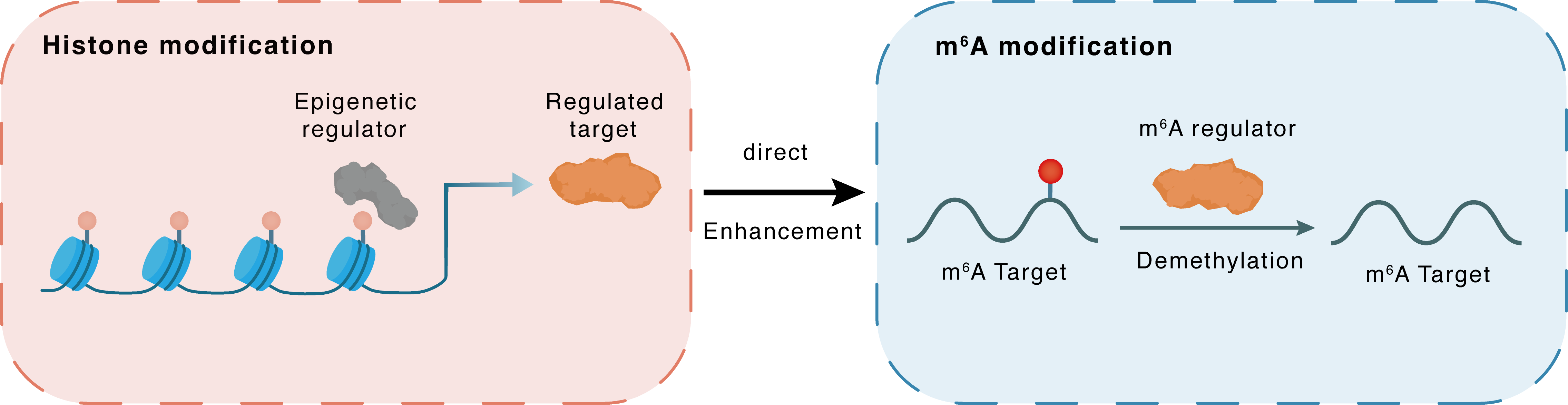 Histone modification
H3K4me3
WDR5
ALKBH5
Direct
Enhancement
m6A modification
SIRT3
SIRT3
ALKBH5
Demethylation
Histone modification
H3K4me3
WDR5
ALKBH5
Direct
Enhancement
m6A modification
SIRT3
SIRT3
ALKBH5
Demethylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | RNA demethylase ALKBH5 (ALKBH5) | ERASER | |||
| m6A Target | NAD-dependent protein deacetylase sirtuin-3, mitochondrial (SIRT3) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | WD repeat-containing protein 5 (WDR5) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 4 trimethylation (H3K4me3) | View Details | |||
| Downstream Gene | ALKBH5 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | E7 increased ALKBH5 expression through WDR5-mediated activation of the Histone H3 lysine 27 acetylation (H3K27ac) histone modifications, as well as post-translational modification mediated by DDX3. ALKBH5-mediated m6A demethylation enhanced the expression of PAK5. The m6A reader YTHDF2 bound to PAK5 mRNA and regulated its stability in an m6A-dependent manner.ALKBH5 demethylates and destabilizes NAD-dependent protein deacetylase sirtuin-3, mitochondrial (SIRT3) in an m6A-IGF2BP1-dependent manner, repressing CESC growth, lipid metabolism and tumorigenesis by downregulating ACC1. | ||||
| Responsed Disease | Cervical cancer | ICD-11: 2C77 | |||
In-vitro Model |
Ca Ski | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1100 | |
| SiHa | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 | ||
| C-33 A | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1094 | ||
| Ect1/E6E7 | Normal | Homo sapiens | CVCL_3679 | ||
| In-vivo Model | The animals were maintained in pathogen-free conditions at 21 ° C ± 2 ° C and 55% ± 5% humidity with free access to food and water. Mice were randomly divided into three groups (n = 8 per group) and received a subcutaneous injection of 2 × 106 stably transfected SiHa cells containing the indicated lentivirus (empty, Lv-ALKBH5, Lv-ALKBH5 + Lv-ACC1) diluted in PBS in the left flank. The mice were sacrificed when tumours were apparent on day 30. Tumour volume was recorded 7, 14, 21 and 28 days after injection with a Vernier calliper. After euthanasia, xenografts were excised from mice and weighed. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| WD repeat-containing protein 5 (WDR5) | 1 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| OICR-9429 | Investigative | [3] | ||
| Synonyms |
1801787-56-3; OICR9429; CHEMBL3798846; N-(4-(4-Methylpiperazin-1-Yl)-3'-(Morpholinomethyl)-[1,1'-Biphenyl]-3-Yl)-6-Oxo-4-(Trifluoromethyl)-1,6-Dihydropyridine-3-Carboxamide; N-[2-(4-methylpiperazin-1-yl)-5-[3-(morpholin-4-ylmethyl)phenyl]phenyl]-6-oxo-4-(trifluoromethyl)-1,6-dihydropyridine-3-carboxamide; GTPL8231; OICR 9429; MolPort-039-101-294; EX-A2417; BCP18185; BDBM50164794; s7833; AKOS025147341; ZINC231558892; SB19642; CS-5776; NCGC00371263-02; AK468854; HY-16993; J3.618.049H
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
References