m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03332
|
[1], [2] | |||
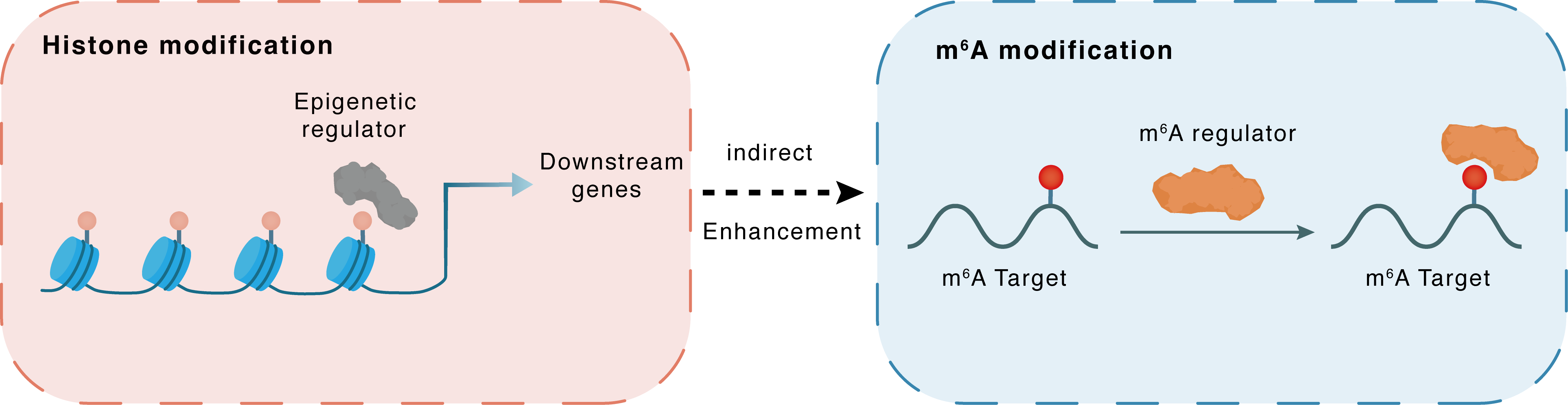 Histone modification
H3K4me3
KDM5A
hsa-miR-495
Indirect
Enhancement
m6A modification
ELAVL1
ELAVL1
YTHDF2
Histone modification
H3K4me3
KDM5A
hsa-miR-495
Indirect
Enhancement
m6A modification
ELAVL1
ELAVL1
YTHDF2
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | |||
| m6A Target | ELAV-like protein 1 (HuR/ELAVL1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Lysine-specific demethylase 5A (KDM5A) | ERASER | View Details | ||
| Regulated Target | Histone H3 lysine 4 trimethylation (H3K4me3) | View Details | |||
| Downstream Gene | hsa-miR-495 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | KDM5A, as a Histone H3 lysine 4 trimethylation (H3K4me3) demethylase, bound to the hsa-miR-495 promoter, which led to inhibition of its transcription and expression. As a target of miR-495, YTHDF2 could inhibit MOB3B expression by recognizing m6A modification of MOB3B mRNA and inducing mRNA degradation. m6A modification levels were markedly upregulated in human PCa tissues due to increased expression of METTL3. METTL3 mediates m6A modification of USP4 mRNA at A2696, and m6A reader protein YTHDF2 binds to and induces degradation of USP4 mRNA by recruiting RNA-binding protein HNRNPD to the mRNA. Decrease of USP4 fails to remove the ubiquitin group from ELAV-like protein 1 (HuR/ELAVL1) protein, resulting in a reduction of ELAVL1 protein. Lastly, downregulation of ELAVL1 in turn increases ARHGDIA expression, promoting migration and invasion of PCa cells. | ||||
| Responsed Disease | Prostate cancer | ICD-11: 2C82 | |||
| Pathway Response | Apoptosis | hsa04210 | |||
| Cell Process | Cell proliferation | ||||
| Cell migration | |||||
| Cell invasion | |||||
| Cell apoptosis | |||||
In-vitro Model |
HNC PC3 | Retromolar trigone squamous cell carcinoma | Homo sapiens | CVCL_C8XA | |
| DU145 | Prostate carcinoma | Homo sapiens | CVCL_0105 | ||
| LNCaP | Prostate carcinoma | Homo sapiens | CVCL_0395 | ||
| In-vivo Model | A total of 1 × 106 PC3 cells or DU145 cells suspended in a mixture of 100 uL PBS and Matrigel were subcutaneously injected into BALB/c nude mice. Tumor weight were measured 2 months after the engraftment. To evaluate the role of METTL3 in tumor metastasis, PC3 cells with or without knockdown of METTL3 were injected into SCID mice through the tail vein (1 × 106 cells per mouse). After eight weeks, mice were sacrificed and their lung tissues were collected for subsequent analyses. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Lysine-specific demethylase 5A (KDM5A) | 21 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| 6EP | Patented | [3] | ||
| Synonyms |
2-{5-[(4-Chloro-2-Methylphenyl)methoxy]-1h-Pyrazol-1-Yl}pyridine-4-Carboxylic Acid; 1613410-75-5; CHEMBL3786952; SCHEMBL15778339; BDBM191600; NCGC00390881-02; QC3611,QC-3611,QC 3611; 2-(5-((4-chloro-2-methylbenzyl)oxy)-1Hpyrazol-1-yl)isonicotinic acid (N19)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| BDBM50158803 | Patented | [4] | ||
| Synonyms |
CHEMBL3787438; SCHEMBL15792889
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| US10022354, Example 151 | Patented | [5] | ||
| Synonyms |
SCHEMBL19513974; CHEMBL4060968; BDBM281211
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.065 nM | |||
| External Link | ||||
| US9714230, 12 | Patented | [3] | ||
| Synonyms |
SCHEMBL15778399; LKBZHRSAENXIOI-UHFFFAOYSA-N; BDBM263942; 2-(5-p-tolyl-1H-pyrazol-1- yl)isonicotinic acid; 2-(5-p-tolyl-1H-pyrazol-1-yl)isonicotinic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 550 nM | |||
| External Link | ||||
| BDBM50158791 | Patented | [6] | ||
| Synonyms |
CHEMBL3786596; SCHEMBL15818867; SCHEMBL19646964
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 550 nM | |||
| External Link | ||||
| US10040779, Example 4 | Patented | [7] | ||
| Synonyms |
SCHEMBL15792083; BDBM277707; 3-[(5-chloro-1-methyl-1H-indazol-3-yl)amino]pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 0.1nM | |||
| External Link | ||||
| US9611221, Example 9 | Patented | [8] | ||
| Synonyms |
3-[(biphenyl-4-ylmethyl)amino]pyridine-4-carboxylic acid; SCHEMBL15286753; RNBCOBWQQCESLL-UHFFFAOYSA-N; BDBM314105
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| US9714230, 46 | Patented | [9] | ||
| Synonyms |
SCHEMBL15778753; MITOFELVTHNBGA-UHFFFAOYSA-N; BDBM263981; 2-(5-(4-bromophenyl)-1H-pyrazol- 1-yl)isonicotinic acid; 2-[5-(4-bromophenyl)-1H-pyrazol-1-yl]pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 10nM | |||
| External Link | ||||
| BDBM50158794 | Patented | [7] | ||
| Synonyms |
CHEMBL3785470; SCHEMBL15792416
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 0.1nM | |||
| External Link | ||||
| BDBM50158703 | Patented | [3] | ||
| Synonyms |
CHEMBL3785832; SCHEMBL15777940
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| AKOS020330656 | Patented | [10] | ||
| Synonyms |
CHEMBL3774545; SCHEMBL15820618; RFUZGPWCXINBNW-UHFFFAOYSA-N; BDBM50153334; ZINC123452149; 3-{[(5-methylthiophen-2-yl)methyl]amino}pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| BDBM50158755 | Patented | [11] | ||
| Synonyms |
CHEMBL3786579; SCHEMBL15778210
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| US10174026, Example 99 | Patented | [12] | ||
| Synonyms |
SCHEMBL16157351; BDBM320432; 2-(pyrrolidin-1-ylcarbonyl)-1H- pyrrolo[3,2-b]pyridine-7- carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| AKOS020330481 | Patented | [8] | ||
| Synonyms |
3-[(4-methoxybenzyl)amino]pyridine-4-carboxylic acid; SCHEMBL4855920; US9611221, Example 7; XKZFNTZMCLZYHZ-UHFFFAOYSA-N; BDBM314103; 3[(4-methoxybenzyl)amino]pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 550 nM | |||
| External Link | ||||
| US10022354, Example 5 | Patented | [5] | ||
| Synonyms |
SCHEMBL17682496; CHEMBL4062756; BDBM281065
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7.4 nM | |||
| External Link | ||||
| NCGC00381656-01 | Patented | [13] | ||
| Synonyms |
CHEMBL4100530; SCHEMBL16157407; BDBM320423; US10174026, Example 88; 2-[(2-chlorophenyl)-propoxy- methyl]-1H-pyrrolo[3,2-b]- pyridine-7-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| US10174026, Example 2 | Patented | [12] | ||
| Synonyms |
SCHEMBL16149258; FEZIKLVLFANZBD-UHFFFAOYSA-N; BDBM320362; 2-phenyl-1H-pyrrolo[3,2-b]pyridine-7-carboxylic acid; 2-phenyl-1H-pyrrolo[3,2-b] pyridine-7-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 550 nM | |||
| External Link | ||||
| US10022354, Example 152 | Patented | [5] | ||
| Synonyms |
CHEMBL4059597; SCHEMBL17682668; BDBM281212
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.083 nM | |||
| External Link | ||||
| US10040779, Example 1 | Patented | [7] | ||
| Synonyms |
SCHEMBL15792304; BDBM277704; 3-[(1-methyl-1H-indazol-3-yl)amino]pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 0.1nM | |||
| External Link | ||||
| 1190312-92-5 | Patented | [12] | ||
| Synonyms |
3-chloro-1H-pyrrolo[3,2-b]pyridine-7-carboxylic acid; 3-CHLORO-4-AZAINDOLE-7-CARBOXYLIC ACID; SCHEMBL16157363; US10174026, Example 1; UAFNSWUBMGTOQA-UHFFFAOYSA-N; BDBM320361; ZINC44713035; 3-chloro-1H-pyrrolo[3,2-b] pyridine-7-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5500 nM | |||
| External Link | ||||
| PBIT | Investigative | [14] | ||
| Synonyms |
2514-30-9; 2-(4-methylphenyl)-1,2-benzisothiazol-3(2H)-one; 2-(4-methylphenyl)-1,2-benzothiazol-3-one; MLS000583746; 2-(p-Tolyl)benzo[d]isothiazol-3(2H)-one; 2-(p-tolyl)-1,2-benzothiazol-3-one; SMR000200989; 2-(4-methylphenyl)-1,2-benzothiazol-3(2H)-one; 1,2-Benzisothiazol-3(2H)-one, 2-(4-methylphenyl)-; 2-(4-methylphenyl)-2,3-dihydro-1,2-benzothiazol-3-one; ChemDiv3_007090; AC1LIP69; cid_935415; SCHEMBL2443755; GTPL7026; CHEMBL1336959; CTK0J4356; BDBM34737; AOB6896; DTXSID10359056; MolPort-002-285-696; HMS2576N21
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2C82: Prostate cancer | 1 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| CC-94676 | Phase 1 | [15] | ||
| External Link | ||||
References