m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03330
|
[1], [2] | |||
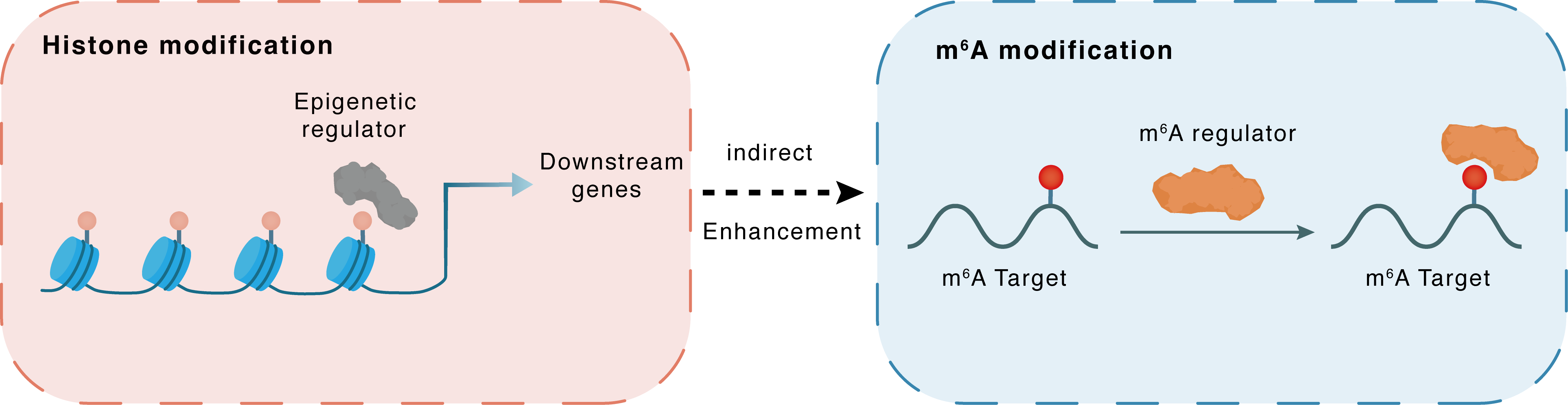 Histone modification
H3K4me3
KDM5A
hsa-miR-495
Indirect
Enhancement
m6A modification
NKX3-1
NKX3-1
YTHDF2
Histone modification
H3K4me3
KDM5A
hsa-miR-495
Indirect
Enhancement
m6A modification
NKX3-1
NKX3-1
YTHDF2
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | |||
| m6A Target | Homeobox protein Nkx-3.1 (NKX3-1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Lysine-specific demethylase 5A (KDM5A) | ERASER | View Details | ||
| Regulated Target | Histone H3 lysine 4 trimethylation (H3K4me3) | View Details | |||
| Downstream Gene | hsa-miR-495 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | KDM5A, as a Histone H3 lysine 4 trimethylation (H3K4me3) demethylase, bound to the hsa-miR-495 promoter, which led to inhibition of its transcription and expression. As a target of miR-495, YTHDF2 could inhibit MOB3B expression by recognizing m6A modification of MOB3B mRNA and inducing mRNA degradation. Knock-down of YTHDF2 or METTL3 significantly induced the expression of LHPP and Homeobox protein Nkx-3.1 (NKX3-1) at both mRNA and protein level with inhibited phosphorylated AKT. YTHDF2 mediates the mRNA degradation of the tumor suppressors LHPP and NKX3-1 in m6A-dependent way to regulate AKT phosphorylation-induced tumor progression in prostate cancer. | ||||
| Responsed Disease | Prostate cancer | ICD-11: 2C82 | |||
| Pathway Response | Oxidative phosphorylation | hsa00190 | |||
| Cell Process | Cell proliferation | ||||
| Cell migration | |||||
| Cell invasion | |||||
| Cell apoptosis | |||||
In-vitro Model |
RWPE-1 | Normal | Homo sapiens | CVCL_3791 | |
| DU145 | Prostate carcinoma | Homo sapiens | CVCL_0105 | ||
| PC-3 | Prostate carcinoma | Homo sapiens | CVCL_0035 | ||
| 22Rv1 | Prostate carcinoma | Homo sapiens | CVCL_1045 | ||
| VCaP | Prostate carcinoma | Homo sapiens | CVCL_2235 | ||
| In-vivo Model | Approximately 2 × 106 PCa cells (PC-3 shNC, shYTHDF2, shMETTL3 cell lines) per mouse suspended in 100 uL PBS were injected in the flank of male BALB/c nude mice (4 weeks old). During the 40-day observation, the tumor size (V = (width2× length × 0.52)) was measured with vernier caliper. Approximately 1.5 × 106 PCa cells suspended in 100 uL of PBS (PC-3 shNC, shYTHDF2, and shMETTL3 cell lines) per mouse were injected into the tail vein of male BALB/c nude mice (4 weeks old). The IVIS Spectrum animal imaging system (PerkinElmer) was used to evaluate the tumor growth (40 days) and whole metastasis conditions (4 weeks and 6 weeks) with 100 uL XenoLight D-luciferin Potassium Salt (15 mg/ml, Perkin Elmer) per mouse. Mice were anesthetized and then sacrificed for tumors and metastases which were sent for further organ-localized imaging as above, IHC staining and hematoxylin-eosin (H&E) staining. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Lysine-specific demethylase 5A (KDM5A) | 21 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| 6EP | Patented | [3] | ||
| Synonyms |
2-{5-[(4-Chloro-2-Methylphenyl)methoxy]-1h-Pyrazol-1-Yl}pyridine-4-Carboxylic Acid; 1613410-75-5; CHEMBL3786952; SCHEMBL15778339; BDBM191600; NCGC00390881-02; QC3611,QC-3611,QC 3611; 2-(5-((4-chloro-2-methylbenzyl)oxy)-1Hpyrazol-1-yl)isonicotinic acid (N19)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| BDBM50158803 | Patented | [4] | ||
| Synonyms |
CHEMBL3787438; SCHEMBL15792889
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| US10022354, Example 151 | Patented | [5] | ||
| Synonyms |
SCHEMBL19513974; CHEMBL4060968; BDBM281211
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.065 nM | |||
| External Link | ||||
| US9714230, 12 | Patented | [3] | ||
| Synonyms |
SCHEMBL15778399; LKBZHRSAENXIOI-UHFFFAOYSA-N; BDBM263942; 2-(5-p-tolyl-1H-pyrazol-1- yl)isonicotinic acid; 2-(5-p-tolyl-1H-pyrazol-1-yl)isonicotinic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 550 nM | |||
| External Link | ||||
| BDBM50158791 | Patented | [6] | ||
| Synonyms |
CHEMBL3786596; SCHEMBL15818867; SCHEMBL19646964
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 550 nM | |||
| External Link | ||||
| US10040779, Example 4 | Patented | [7] | ||
| Synonyms |
SCHEMBL15792083; BDBM277707; 3-[(5-chloro-1-methyl-1H-indazol-3-yl)amino]pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 0.1nM | |||
| External Link | ||||
| US9611221, Example 9 | Patented | [8] | ||
| Synonyms |
3-[(biphenyl-4-ylmethyl)amino]pyridine-4-carboxylic acid; SCHEMBL15286753; RNBCOBWQQCESLL-UHFFFAOYSA-N; BDBM314105
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| US9714230, 46 | Patented | [9] | ||
| Synonyms |
SCHEMBL15778753; MITOFELVTHNBGA-UHFFFAOYSA-N; BDBM263981; 2-(5-(4-bromophenyl)-1H-pyrazol- 1-yl)isonicotinic acid; 2-[5-(4-bromophenyl)-1H-pyrazol-1-yl]pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 10nM | |||
| External Link | ||||
| BDBM50158794 | Patented | [7] | ||
| Synonyms |
CHEMBL3785470; SCHEMBL15792416
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 0.1nM | |||
| External Link | ||||
| BDBM50158703 | Patented | [3] | ||
| Synonyms |
CHEMBL3785832; SCHEMBL15777940
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| AKOS020330656 | Patented | [10] | ||
| Synonyms |
CHEMBL3774545; SCHEMBL15820618; RFUZGPWCXINBNW-UHFFFAOYSA-N; BDBM50153334; ZINC123452149; 3-{[(5-methylthiophen-2-yl)methyl]amino}pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| BDBM50158755 | Patented | [11] | ||
| Synonyms |
CHEMBL3786579; SCHEMBL15778210
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| US10174026, Example 99 | Patented | [12] | ||
| Synonyms |
SCHEMBL16157351; BDBM320432; 2-(pyrrolidin-1-ylcarbonyl)-1H- pyrrolo[3,2-b]pyridine-7- carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| AKOS020330481 | Patented | [8] | ||
| Synonyms |
3-[(4-methoxybenzyl)amino]pyridine-4-carboxylic acid; SCHEMBL4855920; US9611221, Example 7; XKZFNTZMCLZYHZ-UHFFFAOYSA-N; BDBM314103; 3[(4-methoxybenzyl)amino]pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 550 nM | |||
| External Link | ||||
| US10022354, Example 5 | Patented | [5] | ||
| Synonyms |
SCHEMBL17682496; CHEMBL4062756; BDBM281065
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7.4 nM | |||
| External Link | ||||
| NCGC00381656-01 | Patented | [13] | ||
| Synonyms |
CHEMBL4100530; SCHEMBL16157407; BDBM320423; US10174026, Example 88; 2-[(2-chlorophenyl)-propoxy- methyl]-1H-pyrrolo[3,2-b]- pyridine-7-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| US10174026, Example 2 | Patented | [12] | ||
| Synonyms |
SCHEMBL16149258; FEZIKLVLFANZBD-UHFFFAOYSA-N; BDBM320362; 2-phenyl-1H-pyrrolo[3,2-b]pyridine-7-carboxylic acid; 2-phenyl-1H-pyrrolo[3,2-b] pyridine-7-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 550 nM | |||
| External Link | ||||
| US10022354, Example 152 | Patented | [5] | ||
| Synonyms |
CHEMBL4059597; SCHEMBL17682668; BDBM281212
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.083 nM | |||
| External Link | ||||
| US10040779, Example 1 | Patented | [7] | ||
| Synonyms |
SCHEMBL15792304; BDBM277704; 3-[(1-methyl-1H-indazol-3-yl)amino]pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 0.1nM | |||
| External Link | ||||
| 1190312-92-5 | Patented | [12] | ||
| Synonyms |
3-chloro-1H-pyrrolo[3,2-b]pyridine-7-carboxylic acid; 3-CHLORO-4-AZAINDOLE-7-CARBOXYLIC ACID; SCHEMBL16157363; US10174026, Example 1; UAFNSWUBMGTOQA-UHFFFAOYSA-N; BDBM320361; ZINC44713035; 3-chloro-1H-pyrrolo[3,2-b] pyridine-7-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5500 nM | |||
| External Link | ||||
| PBIT | Investigative | [14] | ||
| Synonyms |
2514-30-9; 2-(4-methylphenyl)-1,2-benzisothiazol-3(2H)-one; 2-(4-methylphenyl)-1,2-benzothiazol-3-one; MLS000583746; 2-(p-Tolyl)benzo[d]isothiazol-3(2H)-one; 2-(p-tolyl)-1,2-benzothiazol-3-one; SMR000200989; 2-(4-methylphenyl)-1,2-benzothiazol-3(2H)-one; 1,2-Benzisothiazol-3(2H)-one, 2-(4-methylphenyl)-; 2-(4-methylphenyl)-2,3-dihydro-1,2-benzothiazol-3-one; ChemDiv3_007090; AC1LIP69; cid_935415; SCHEMBL2443755; GTPL7026; CHEMBL1336959; CTK0J4356; BDBM34737; AOB6896; DTXSID10359056; MolPort-002-285-696; HMS2576N21
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2C82: Prostate cancer | 1 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| CC-94676 | Phase 1 | [15] | ||
| External Link | ||||
References