m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03276
|
[1], [2] | |||
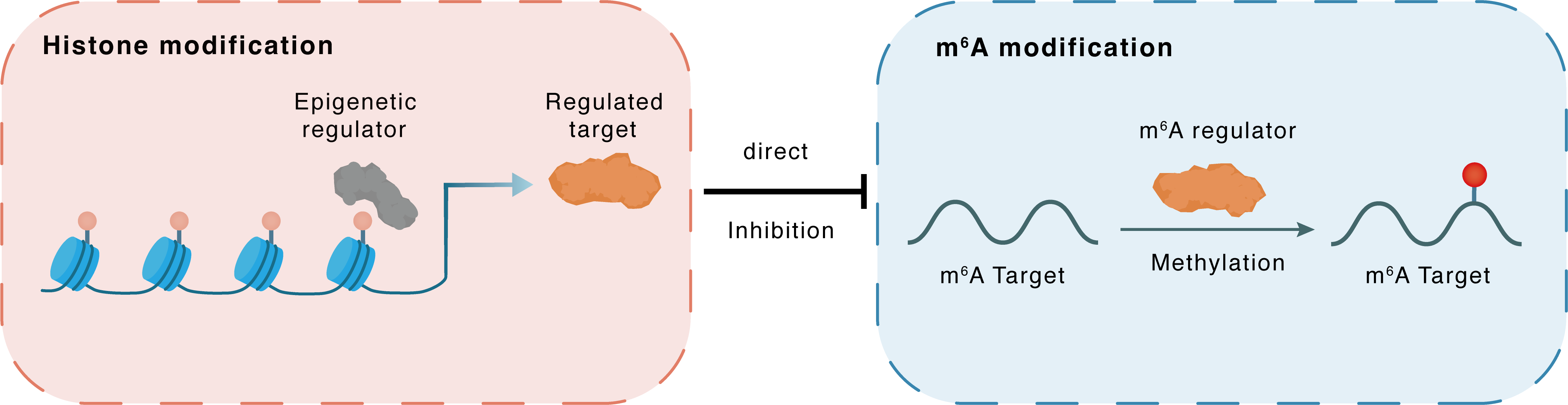 Histone modification
H3K4me3
KDM5C
METTL14
Direct
Inhibition
m6A modification
MIR375
MIR375
METTL14
Methylation
Histone modification
H3K4me3
KDM5C
METTL14
Direct
Inhibition
m6A modification
MIR375
MIR375
METTL14
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 14 (METTL14) | WRITER | |||
| m6A Target | microRNA 375 (MIR375) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Lysine-specific demethylase 5C (KDM5C) | ERASER | View Details | ||
| Regulated Target | Histone H3 lysine 4 trimethylation (H3K4me3) | View Details | |||
| Downstream Gene | METTL14 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Inhibition | |||
| Crosstalk Mechanism | histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | Both the univariate and multivariate Cox regression analysis indicated that METTL14 was an independent prognostic factor in CRC. Moreover, KDM5C-mediated demethylation of Histone H3 lysine 36 trimethylation (H3K36me3) in the promoter of METTL14 inhibited METTL14 transcription. METTL14 suppressed Colorectal cancer cell growth, migration, and invasion via the microRNA 375 (MIR375)/YAP1 and miR-375/SP1 pathways. | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | Hippo signaling pathway | hsa04390 | |||
| Cell Process | Cell growth and metastasis | ||||
In-vitro Model |
NCM460 | Normal | Homo sapiens | CVCL_0460 | |
| HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| HCT 8 | Colon adenocarcinoma | Homo sapiens | CVCL_2478 | ||
| SW620 | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| SW480 | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| HT29 | Colon cancer | Mus musculus | CVCL_A8EZ | ||
| DLD-1 | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| In-vivo Model | All animal experiments were approved by the animal care Committee of Nanjing First Hospital, Nanjing Medial University (acceptance No. SYXK 20160006). 2 × 106 transfected HCT116 cells in 0.2 ml PBS were injected into the tail vein of nude mice which were randomly divided into nine groups (eight mice per group). After 2 months of injection, mice were sacrificed, and their lungs were removed and stained by Hematoxylin and Eosin (HE) Staining. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Lysine-specific demethylase 5C (KDM5C) | 2 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PBIT | Investigative | [3] | ||
| Synonyms |
2514-30-9; 2-(4-methylphenyl)-1,2-benzisothiazol-3(2H)-one; 2-(4-methylphenyl)-1,2-benzothiazol-3-one; MLS000583746; 2-(p-Tolyl)benzo[d]isothiazol-3(2H)-one; 2-(p-tolyl)-1,2-benzothiazol-3-one; SMR000200989; 2-(4-methylphenyl)-1,2-benzothiazol-3(2H)-one; 1,2-Benzisothiazol-3(2H)-one, 2-(4-methylphenyl)-; 2-(4-methylphenyl)-2,3-dihydro-1,2-benzothiazol-3-one; ChemDiv3_007090; AC1LIP69; cid_935415; SCHEMBL2443755; GTPL7026; CHEMBL1336959; CTK0J4356; BDBM34737; AOB6896; DTXSID10359056; MolPort-002-285-696; HMS2576N21
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| IOX2 | Investigative | [4] | ||
| Synonyms |
compound 6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B91: Colorectal cancer | 25 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Retifanlimab | Approved | [5] | ||
| Synonyms |
INCMGA0012; Retifanlimab
Click to Show/Hide
|
|||
| External Link | ||||
| Aflibercept | Approved | [6] | ||
| Synonyms |
Ziv-Aflibercept; Zaltrap (TN); VEGF Trap; VEGF Trap-Eye
Click to Show/Hide
|
|||
| External Link | ||||
| Regorafenib | Approved | [7] | ||
| Synonyms |
755037-03-7; BAY 73-4506; Regorafenibum; Stivarga; 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; BAY73-4506; Regorafenib (BAY 73-4506); UNII-24T2A1DOYB; 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide; BAY-73-4506; 24T2A1DOYB; CHEMBL1946170; CHEBI:68647; Stivarga (TN); BAY73-4506 hydrochloride; Regorafenib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [8] | ||
| Synonyms |
Bevacizumab (ophthalmic slow-release tissue tablet)
Click to Show/Hide
|
|||
| External Link | ||||
| SYM-004 | Phase 3 | [8] | ||
| Synonyms |
Chimeric IgG1 antibody 1024 (cancer), Symphogen; Chimeric IgG1 antibody 992 (cancer), Symphogen; Chimeric IgG1 antibodies992 + 1024 (cancer), Symphogen
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab + Erlotinib | Phase 3 | [9] | ||
| External Link | ||||
| CPI-613 | Phase 3 | [8] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [6] | ||
| External Link | ||||
| AlloStim | Phase 2/3 | [10] | ||
| Synonyms |
AlloStim (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Sibrotuzumab | Phase 2 | [11] | ||
| External Link | ||||
| CV301 | Phase 2 | [12] | ||
| External Link | ||||
| Efatutazone | Phase 2 | [13] | ||
| Synonyms |
Inolitazone; 223132-37-4; 5-[[4-[[6-(4-amino-3,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-yl]methoxy]phenyl]methyl]-2,4-Thiazolidinedione; Efatutazone [INN]; RS5444; CS-7017; SCHEMBL3246054; CHEMBL3545280; JCYNMRJCUYVDBC-UHFFFAOYSA-N; Efatutazone;CS-7017;RS5444; BCP07478; AKOS030526729; DB11894; CS-0778; KB-77905; DA-07988; HY-14792; QC-10456; 4CA-1384; FT-0737589; 5-[4-[6-(4-amino-3 ,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-ylmethoxy]benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| LOR-2040 | Phase 2 | [14] | ||
| External Link | ||||
| RG7221 | Phase 2 | [15] | ||
| External Link | ||||
| PEG-SN38 | Phase 2 | [16] | ||
| Synonyms |
EZN-2208
Click to Show/Hide
|
|||
| External Link | ||||
| MEGF0444A | Phase 2 | [17] | ||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [18] | ||
| External Link | ||||
| AB928 | Phase 1/2 | [19] | ||
| External Link | ||||
| MGD007 | Phase 1 | [15] | ||
| External Link | ||||
| BNC-101 | Phase 1 | [20] | ||
| External Link | ||||
| Navicixizumab | Phase 1 | [8] | ||
| External Link | ||||
| RG7160 | Discontinued in Phase 2 | [21] | ||
| External Link | ||||
| Nimesulide | Terminated | [22] | ||
| Synonyms |
51803-78-2; N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide; Mesulid; Flogovital; Sulidene; Nimed; R-805; 4-NITRO-2-PHENOXYMETHANESULFONANILIDE; Nisulid; Nimesulidum [INN-Latin]; Nimesulida [INN-Spanish]; R 805; UNII-V4TKW1454M; 4-Nitro-2-phenoxy-methanesulfonanilide; 4'-Nitro-2'-phenoxymethanesulfonanilide; Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-; EINECS 257-431-4; 4'-Nitro-2'-phenoxymethansulfonanilid; BRN 2421175; CHEMBL56367; MLS000069680; V4TKW1454M; Methanesulfonanilide, 4'-nitro-2'-phenoxy-; CHEBI:44445; Dulanermin
Click to Show/Hide
|
|||
| External Link | ||||
| Saracatinib | Phase 2 | [23] | ||
| External Link | ||||
| G3139 + Irinotecan | Investigative | [24] | ||
| External Link | ||||
References