m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03209
|
[1] | |||
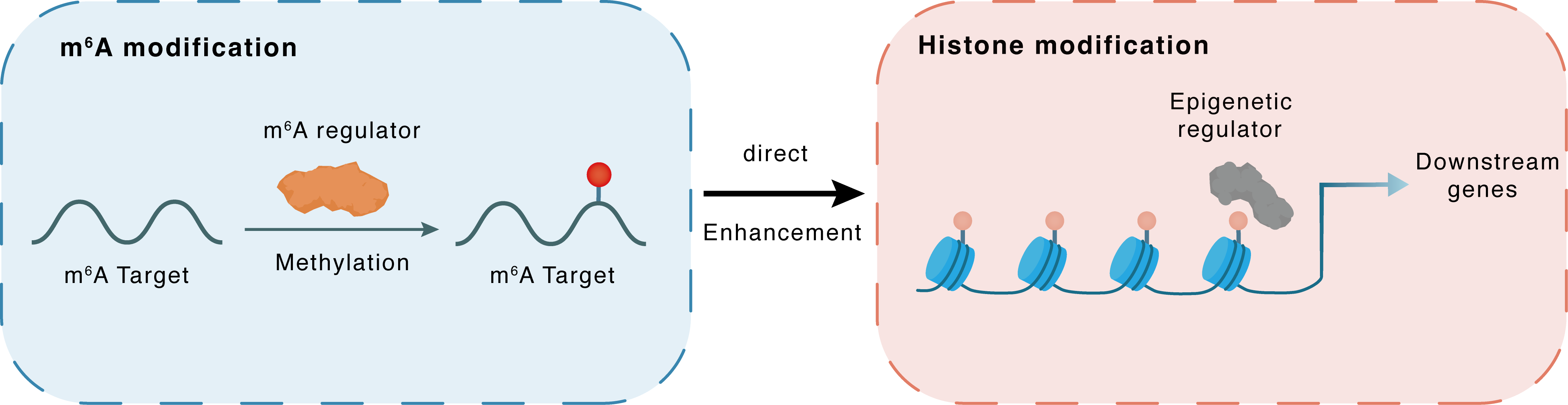 m6A modification
NSD2
NSD2
METTL3
Methylation
m6A modification
NSD2
NSD2
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Histone modification
H3K36me2
NSD2
Downstream Gene : m6A sites
Direct
Enhancement
Histone modification
H3K36me2
NSD2
Downstream Gene
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Histone-lysine N-methyltransferase NSD2 (NSD2) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone-lysine N-methyltransferase NSD2 (NSD2) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 36 dimethylation (H3K36me2) | View Details | |||
| Crosstalk Relationship | m6A → Histone modification | Enhancement | |||
| Crosstalk Mechanism | m6A modification impacts directly histone modification through modulating the expression level of histone-associated enzymes | ||||
| Crosstalk Summary | METTL3 promoted m6A modification of Histone-lysine N-methyltransferase NSD2 (NSD2) mRNA and enhanced its stability by YTHDF1. Moreover, NSD2 catalyzes Histone H3 lysine 36 dimethylation (H3K36me2) , playing a crucial role in chromatin regulation and transcriptional control. | ||||
| Responsed Disease | Diabetic nephropathy | ICD-11: GB61.Z | |||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| GB61: Chronic kidney disease | 15 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Finerenone | Approved | [2] | ||
| Synonyms |
UNII-DE2O63YV8R; BAY 94-8862; 1050477-31-0; BAY94-8862; DE2O63YV8R; Finerenone [USAN:INN]; Finerenone (JAN/USAN/INN); SCHEMBL8157011; GTPL8678; DTXSID10146928; J3.584.878I; D10633; 1,6-Naphthyridine-3-carboxamide, 4-(4-cyano-2-methoxyphenyl)-5-ethoxy-1,4-dihydro-2,8-dimethyl-, (4S)-;1,6-Naphthyridine-3-carboxamide, 4-(4-cyano-2-methoxyphenyl)-5-ethoxy-1,4-dihydro-2,8-dimethyl-, (4S)-; 1,6-Naphthyridine-3-carboxamide, 4-(4-cyano-2-methoxyphenyl)-5-ethoxy-1,4-dihydro-2,8-dimethyl-, (4S)-; (4S)-4-(4-cyano-2-metho
Click to Show/Hide
|
|||
| External Link | ||||
| Doxercalciferol | Approved | [3] | ||
| Synonyms |
Doxcercalciferol; Hectorol; Doxercalciferol [INN]; TSA 840; BCI-101; Doxercalciferol (INN); Hectorol (TN); (1R,3S,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(E,2R,5R)-5,6-dimethylhept-3-en-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol; (5Z,7E,22E)-9,10-Secoergosta-5,7,10(19),22-tetraene-1alpha,3beta-diol; 1-Hydroxyergocalciferol; 1-alpha-Hydroxyvitamin D2; 1alpha-Hydroxyergocalciferol; 1alpha-OH-D2; 9,10-Secoergosta-5,7,10(19),22-tetraene-1,3-diol,(1-alpha,3-beta,5Z,7E,22E)
Click to Show/Hide
|
|||
| External Link | ||||
| Ferumoxytol | Approved | [4] | ||
| Synonyms |
MAGNETITE; Magnetic oxide; Ferrosoferric oxide; Magnetite (Fe3O4); Magnetic Black; Iron Black; Fenosoferric oxide; Black Iron BM; Meramec M 25; Black Gold F 89; RB-BL; 11557 Black; CCRIS 4376; H 3S; EPT 500; EINECS 215-169-8; KN 320; 1309-38-2; iron(ii; ferro ferric oxide; ferric ferrous oxide; Iron ores, magnetite; Ferumoxytol [USAN]; Eisen(II,III)-oxid; KBC 100 (mineral); Code 7228; CHEBI:50821; 1317-61-9 (Parent); LS-88610; 174794-75-3; 122303-97-3; 90577-09-6; 73904-98-0; 151820-32-5; 137263-94-6; 124364-57-4
Click to Show/Hide
|
|||
| External Link | ||||
| Ferric citrate | Approved | [5] | ||
| Synonyms |
Nephoxil; Serene; Zerenex; JTT-751; KRX-0502; PBF-1681; Hyperphosphatemia therapy, Panion/Keryx
Click to Show/Hide
|
|||
| External Link | ||||
| REACT | Phase 3 | [6] | ||
| External Link | ||||
| US-APR2020 | Phase 2/3 | [7] | ||
| External Link | ||||
| ALLN-346 | Phase 2 | [8] | ||
| External Link | ||||
| Runcaciguat | Phase 2 | [9] | ||
| Synonyms |
(3S)-3-(4-Chloro-3-(((2S,3R)-2-(4-chlorophenyl-4,4,4- trifluoro-3-methylbutanoyl)amino)phenyl)-3- cyclopropylpropanoic acid; (3S)-3-(4-chloro-3-{[(2S,3R)-2-(4-chlorophenyl)-4,4,4-trifluoro-3-methylbutanoyl]amino}phenyl)-3-cyclopropylpropanoic acid; (3S)-3-[4-chloro-3-[[(2S,3R)-2-(4-chlorophenyl)-4,4,4-trifluoro-3-methylbutanoyl]amino]phenyl]-3-cyclopropylpropanoic acid; 1402936-61-1; 5EZ01YDT5S; AC-37098; AKOS040742586; BAY 1101042; BAY1101042; BAY-1101042; BENZENEPROPANOIC ACID, 4-CHLORO-3-(((2S,3R)-2-(4-CHLOROPHENYL)-4,4,4-TRIFLUORO-3-METHYL-1-OXOBUTYL)AMINO)-.BETA.-CYCLOPROPYL-, (.BETA.S)-; Benzenepropanoic acid, 4-chloro-3-(((2S,3R)-2-(4-chlorophenyl)-4,4,4-trifluoro-3-methyl-1-oxobutyl)amino)-beta-cyclopropyl-, (betaS)-; CHEMBL4650322; compound 45 [PMID: 33872507]; CS-0086784; GTPL12359; HY-109136; MS-29070; Runcaciguat; Runcaciguat [INN]; SCHEMBL20075857; UNII-5EZ01YDT5S; XZ7
Click to Show/Hide
|
|||
| External Link | ||||
| GCS-100 | Phase 2 | [10] | ||
| External Link | ||||
| Neo-Kidney Augment | Phase 2 | [11] | ||
| External Link | ||||
| LY-2623091 | Phase 2 | [12] | ||
| Synonyms |
Chronic renal disease therapy, Eli Lilly
Click to Show/Hide
|
|||
| External Link | ||||
| AZD1772//RDX5791 | Phase 2 | [13] | ||
| External Link | ||||
| LY3016859 | Phase 1/2 | [14] | ||
| Synonyms |
TGF-alpha.epiregulin mAb
Click to Show/Hide
|
|||
| External Link | ||||
| ION532 | Phase 1 | [15] | ||
| Synonyms |
AZD2373
Click to Show/Hide
|
|||
| External Link | ||||
| MEDI8367 | Phase 1 | [16] | ||
| External Link | ||||
References