m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03161
|
[1] | |||
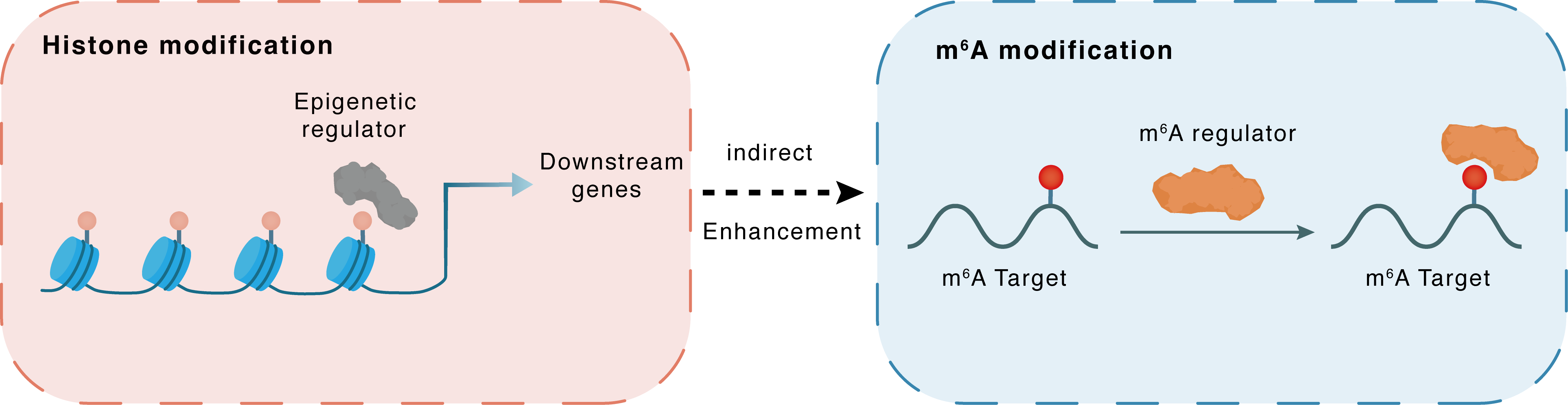 Histone modification
H3K4me3
KMT2A
METTL3
Indirect
Enhancement
m6A modification
ATG4a
ATG4a
YTHDF2
Histone modification
H3K4me3
KMT2A
METTL3
Indirect
Enhancement
m6A modification
ATG4a
ATG4a
YTHDF2
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | |||
| m6A Target | Cysteine protease ATG4A (ATG4A) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone-lysine N-methyltransferase 2A (KMT2A) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 4 trimethylation (H3K4me3) | View Details | |||
| Downstream Gene | METTL3 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | KMT2A-mediated Histone H3 lysine 4 trimethylation (H3K4me3) regulates the autophagy-GATA4 axis through METTL3-mediated m6A modification of Cysteine protease ATG4A (ATG4A) in a YTHDF2 dependent manner to promote NPCs senescence and IVDD progression | ||||
| Responsed Disease | Intervertebral disc degeneration | ICD-11: FA80 | |||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| FA80: Intervertebral disc degeneration | 3 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Rexlemestrocel-L | Phase 3 | [2] | ||
| Synonyms |
MPC-150-M
Click to Show/Hide
|
|||
| External Link | ||||
| CybroCell | Phase 1/2 | [3] | ||
| External Link | ||||
| IDCT | Phase 1/2 | [4] | ||
| Synonyms |
rebonuputemcel
Click to Show/Hide
|
|||
| External Link | ||||
References