m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03148
|
[1] | |||
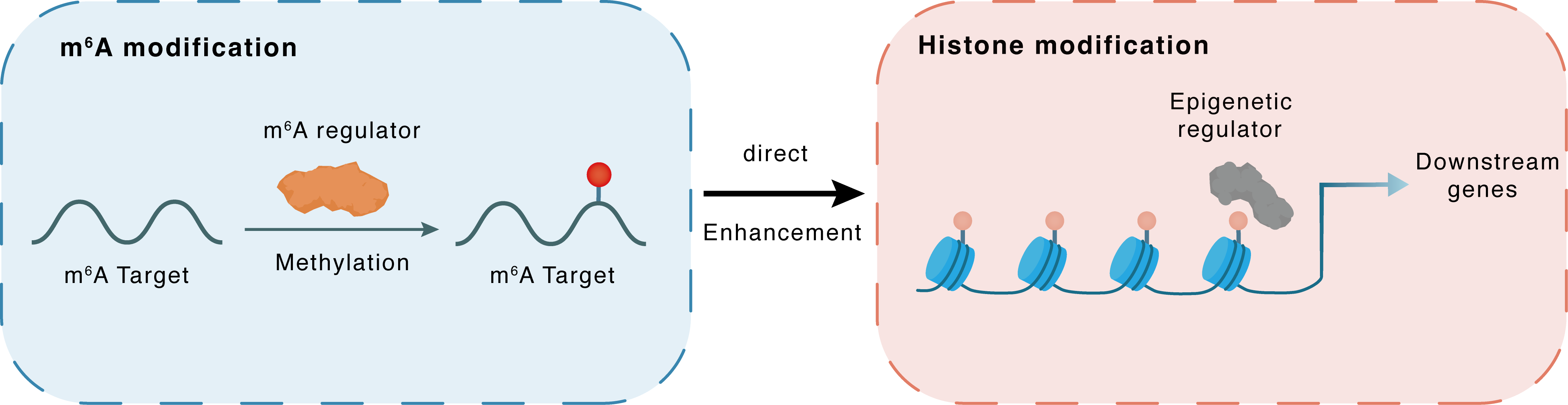 m6A modification
HDAC6
HDAC6
METTL3
Methylation
m6A modification
HDAC6
HDAC6
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Histone modification
HisMod sites
HDAC6
Downstream Gene : m6A sites
Direct
Enhancement
Histone modification
HisMod sites
HDAC6
Downstream Gene
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Protein deacetylase HDAC6 (HDAC6) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Protein deacetylase HDAC6 (HDAC6) | ERASER | View Details | ||
| Regulated Target | Histone acetylation | View Details | |||
| Crosstalk Relationship | m6A → Histone modification | Enhancement | |||
| Crosstalk Mechanism | m6A modification impacts directly histone modification through modulating the expression level of histone-associated enzymes | ||||
| Crosstalk Summary | Depletion of METTL3-mediated m6A modification leads to abnormally elongated cilia via suppressing Protein deacetylase HDAC6 (HDAC6)-dependent deacetylation of axonemal alpha-tubulin, ultimately attenuating cell growth and cervical cancer development. | ||||
In-vitro Model |
HeLa | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| SiHa | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 | ||
| In-vivo Model | To construct the subcutaneous xenograft model, ~1 × 106 HeLa cells suspended in 50% Matrigel in DMEM were subcutaneously injected into the right flanks of the mice. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Protein deacetylase HDAC6 (HDAC6) | 171 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Citarinostat | Phase 1 | [2] | ||
| Synonyms |
ACY-241; 1316215-12-9; HDAC-IN-2; 2-((2-Chlorophenyl)(phenyl)amino)-N-(7-(hydroxyamino)-7-oxoheptyl)pyrimidine-5-carboxamide; UNII-441P620G3P; 441P620G3P; Citarinostat [USAN]; Citarinostat (USAN); 2-(N-(2-chlorophenyl)anilino)-N-[7-(hydroxyamino)-7-oxoheptyl]pyrimidine-5-carboxamide; 2-((2-Chlorophenyl)phenylamino)-N-(7-(hydroxyamino)-7-oxoheptyl)-5-pyrimidinecarboxamide; 2-[(2-Chlorophenyl)phenylamino]-N-[7-(hydroxyamino)-7-oxoheptyl]-5-pyrimidinecarboxamide; Citarinostat (ACY-241); SCHEMBL2225863; GTPL942
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.6 nM | |||
| External Link | ||||
| KA2507 | Phase 1 | [2] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Sulfonamide derivative 16 | Patented | [3] | ||
| Synonyms |
PMID29886770-Compound-Figure6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID29671355-Compound-15 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| PMID29671355-Compound-14 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 100 nM | |||
| External Link | ||||
| PMID29671355-Compound-16 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| PMID28092474-Compound-33d | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32u | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-33a | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32a | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32j | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32z | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 500 nM; IC50 <= 1000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32g | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-34c | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32x | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-33b | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32b | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32o | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-33g | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-33j | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-33p | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-33m | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32v | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-24 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.26 nM | |||
| External Link | ||||
| PMID28092474-Compound-34b | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-33e | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32t | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32c | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-33i | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32r | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32h | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-65a | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 7.9 nM | |||
| External Link | ||||
| PMID28092474-Compound-32y | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-33h | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-38a | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 100 nM | |||
| External Link | ||||
| PMID28092474-Compound-33f | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| Diaryl amine derivative 3 | Patented | [5] | ||
| Synonyms |
PMID28092474-Compound-11
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 9 nM | |||
| External Link | ||||
| PMID28092474-Compound-33c | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-39 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| PMID29671355-Compound-19 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32e | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32m | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32p | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32d | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-74 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 433500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32n | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-33k | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32k | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-34a | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-22 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 56 nM | |||
| External Link | ||||
| PMID29671355-Compound-26 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.9 nM | |||
| External Link | ||||
| PMID28092474-Compound-33o | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32f | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| Diaryl amine derivative 2 | Patented | [5] | ||
| Synonyms |
PMID28092474-Compound-10
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 45 nM | |||
| External Link | ||||
| PMID28092474-Compound-33l | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32i | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID28092474-Compound-32q | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID29671355-Compound-18 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-27 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 66 nM | |||
| External Link | ||||
| PMID28092474-Compound-32l | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| Diaryl amine derivative 4 | Patented | [5] | ||
| Synonyms |
PMID28092474-Compound-9
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| PMID28092474-Compound-32s | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-38b | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1.76 nM | |||
| External Link | ||||
| PMID29671355-Compound-28 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 107 nM | |||
| External Link | ||||
| PMID29671355-Compound-73 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1000 nM | |||
| External Link | ||||
| PMID29671355-Compound-13 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 100 nM | |||
| External Link | ||||
| PMID29671355-Compound-11 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1.5 nM | |||
| External Link | ||||
| PMID29671355-Compound-9 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1.7 nM | |||
| External Link | ||||
| PMID29671355-Compound-8 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6 nM | |||
| External Link | ||||
| PMID29671355-Compound-61 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1000000 nM | |||
| External Link | ||||
| PMID29671355-Compound-23 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3.57 nM | |||
| External Link | ||||
| PMID29671355-Compound-44 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 150 nM | |||
| External Link | ||||
| PMID29671355-Compound-56 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 30000 nM | |||
| External Link | ||||
| PMID29671355-Compound-67 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 9000 nM | |||
| External Link | ||||
| PMID29671355-Compound-31 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 4.1 nM | |||
| External Link | ||||
| PMID29671355-Compound-21 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PMID29671355-Compound-62 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1000000 nM | |||
| External Link | ||||
| PMID29671355-Compound-43 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.8 nM | |||
| External Link | ||||
| PMID29671355-Compound-25 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.958 nM | |||
| External Link | ||||
| IKH-25 | Investigative | [6] | ||
| Synonyms |
HDAC-6 inhibitors (cancer), Ikerchem
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (S)-2-Amino-N-cyclopentyl-7-mercaptoheptanamide | Investigative | [7] | ||
| Synonyms |
thiolate analogue, 26a; CHEMBL235911; SCHEMBL16338810; BDBM19139
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Tubacin | Investigative | [8] | ||
| Synonyms |
537049-40-4; AC1O7Y2P; CHEMBL356769; 1350555-93-9; N1-(4-((2R,4R,6S)-4-(((4,5-Diphenyloxazol-2-yl)thio)methyl)-6-(4-(hydroxymethyl)phenyl)-1,3-dioxan-2-yl)phenyl)-N8-hydroxyoctanediamide; Tubacin (BML-GR362); Octanediamide, N1-(4-((2R,4R,6S)-4-(((4,5-diphenyl-2-oxazolyl)thio)methyl)-6-(4-(hydroxymethyl)phenyl)-1,3-dioxan-2-yl)phenyl)-N8-hydroxy-, rel-; N-[4-[(2R,4R,6S)-4-[[(4,5-Diphenyl-2-oxazolyl)thio]methyl]-6-[4-(hydroxymethyl)phenyl]-1,3-dioxan-2-yl]phenyl]-N'-hydroxyoctanediamide; SCHEMBL4741166
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 0.9 nM | |||
| External Link | ||||
| ST-2741 | Investigative | [9] | ||
| Synonyms |
CHEMBL564876; SCHEMBL1306499; BDBM50297444
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6 nM | |||
| External Link | ||||
| N-(quinolin-8-yl)-6-(sulfamoylamino)hexanamide | Investigative | [10] | ||
| Synonyms |
CHEMBL507114; SCHEMBL5330834
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 390 nM | |||
| External Link | ||||
| PMID19111466C7d | Investigative | [11] | ||
| Synonyms |
GTPL7056; BDBM50255914
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 40 nM | |||
| External Link | ||||
| UCL-67022 | Investigative | [6] | ||
| Synonyms |
HDAC inhibitor (multiple myeloma), ST Barts/UCL
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID20947351C16 | Investigative | [12] | ||
| Synonyms |
GTPL7057; BDBM50331106
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 26 nM | |||
| External Link | ||||
| N-(quinolin-6-yl)-6-(sulfamoylamino)hexanamide | Investigative | [10] | ||
| Synonyms |
CHEMBL454438; SCHEMBL5359458; CGIYOYXFUHZSMD-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 490 nM | |||
| External Link | ||||
| NQN-1 | Investigative | [13] | ||
| Synonyms |
PPM-18; NSC 73233
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5540 nM | |||
| External Link | ||||
| N-phenyl-6-(sulfamoylamino)hexanamide | Investigative | [10] | ||
| Synonyms |
CHEMBL474097; SCHEMBL5326902
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1500 nM | |||
| External Link | ||||
| N-(biphenyl-3-yl)-6-(sulfamoylamino)hexanamide | Investigative | [10] | ||
| Synonyms |
CHEMBL475301; SCHEMBL5327672
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1100 nM | |||
| External Link | ||||
| N-[5-(Formyl-hydroxy-amino)-pentyl]-benzamide | Investigative | [14] | ||
| Synonyms |
CHEMBL337584; TWZ-109
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4300 nM | |||
| External Link | ||||
| N-(quinolin-3-yl)-6-(sulfamoylamino)hexanamide | Investigative | [10] | ||
| Synonyms |
CHEMBL475714; SCHEMBL5458681
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 710 nM | |||
| External Link | ||||
| 6-(9H-carbazol-9-yl)-N-hydroxyhexanamide | Investigative | [12] | ||
| Synonyms |
CHEMBL1290142; A1-02262; SCHEMBL1004139; SOMDVJCUFVPZKM-UHFFFAOYSA-N; BDBM50331109; 9H-Carbazole-9-hexanamide, N-hydroxy-; 6-Carbazol-9-ylhexanoic acid hydroxyamide; US8748451, 1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 41 nM | |||
| External Link | ||||
| N1-(biphenyl-3-yl)-N8-hydroxyoctanediamide | Investigative | [10] | ||
| Synonyms |
CHEMBL473270
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 9 nM | |||
| External Link | ||||
| NILTUBACIN | Investigative | [15] | ||
| Synonyms |
Probes1_000174; Probes2_000141; AC1O7Y2N; CHEMBL1213539; SCHEMBL14476139; DIOX-H_003550; 8-[4-[(2R,4R,6S)-4-[(4,5-diphenyl-1,3-oxazol-2-yl)sulfanylmethyl]-6-[4-(hydroxymethyl)phenyl]-1,3-dioxan-2-yl]anilino]-8-oxooctanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2200 nM | |||
| External Link | ||||
| nexturastat A | Investigative | [16] | ||
| Synonyms |
S7473
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3.8 nM | |||
| External Link | ||||
| N1-hydroxy-N8-(4-phenylthiazol-2-yl)octanediamide | Investigative | [17] | ||
| Synonyms |
CHEMBL511212; BDBM50258645
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| droxinostat | Investigative | [18] | ||
| Synonyms |
NS-41080; NS41080
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-L-A1in-L-Ala-D-Pro-) | Investigative | [19] | ||
| Synonyms |
CHEMBL393260
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-mercapto-N-(4-phenylthiazol-2-yl)heptanamide | Investigative | [7] | ||
| Synonyms |
CHEMBL419758; NCH-31; JMC505425 Compound 7; BDBM19131; 7-mercapto-N-(4-phenyl-2-thiazolyl)heptanamide; N-(4-phenyl-1,3-thiazol-2-yl)-7-sulfanylheptanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 230 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-D-A1in-L-Ala-D-Pro-) | Investigative | [19] | ||
| Synonyms |
CHEMBL390991
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-A2in-L-Ala-D-Pro-) | Investigative | [19] | ||
| Synonyms |
CHEMBL394261
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ph5-D-Pro-) | Investigative | [19] | ||
| Synonyms |
CHEMBL391384
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-D-2MePhe-L-Ala-D-Pro-) | Investigative | [19] | ||
| Synonyms |
CHEMBL393261
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N1-(biphenyl-4-yl)-N8-hydroxyoctanediamide | Investigative | [17] | ||
| Synonyms |
CHEMBL512644; SCHEMBL8226957
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| santacruzamate A | Investigative | [20] | ||
| Synonyms |
CAY10683
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 433 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ser(Bzl)-D-Pro-) | Investigative | [19] | ||
| Synonyms |
CHEMBL241555
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Phg-D-Pro-) | Investigative | [19] | ||
| Synonyms |
CHEMBL428737
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ala-D-Tic-) | Investigative | [19] | ||
| Synonyms |
CHEMBL238587
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ala-D-Pro-) | Investigative | [19] | ||
| Synonyms |
CHEMBL238596; BDBM50222727
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 430 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ser-D-Pro-) | Investigative | [19] | ||
| Synonyms |
CHEMBL393961
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ph4-D-Pro-) | Investigative | [19] | ||
| Synonyms |
CHEMBL391383
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-L-2MePhe-L-Ala-D-Pro-) | Investigative | [19] | ||
| Synonyms |
CHEMBL393464
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Phe-D-Pro-) | Investigative | [19] | ||
| Synonyms |
CHEMBL238829; BDBM50222732
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 404 nM | |||
| External Link | ||||
| 4-Phenylbutyrohydroxamic acid | Investigative | [15] | ||
| Synonyms |
N-Hydroxy-4-phenylbutanamide; 32153-46-1; NSC131300; UNII-QX182FOM5S; QX182FOM5S; 4-phenylbutanehydroxamic acid; CHEMBL55895; Benzenebutanamide, N-hydroxy-; NSC 131300; AC1Q7DIW; AC1L5RDX; Phenylbutyrylhydroxamic Acid; AC1Q5QD1; N-Hydroxy-4-phenyl-butyramide; 4-Phenylbutyryl hydroxamic acid; SCHEMBL1350853; CTK4G8310; DTXSID60185943; MolPort-011-492-164; UPHXPXYRKPCXHK-UHFFFAOYSA-N; ZINC4962622; STL301752; BDBM50015142; AKOS009266186; MCULE-9765156954; NSC-131300; NE28489; BCB03_000829; EN300-68596
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 150 nM | |||
| External Link | ||||
| 8-Oxo-8-phenyl-octanoic acid hydroxyamide | Investigative | [21] | ||
| Synonyms |
CHEMBL95959; SCHEMBL3383197; N-hydroxy-8-oxo-8-phenyloctanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ST-3050 | Investigative | [22] | ||
| Synonyms |
CHEMBL472631; SCHEMBL3445133; SCHEMBL3445139; BDBM50278222
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 303 nM | |||
| External Link | ||||
| Octanedioic acid bis-hydroxyamide | Investigative | [23] | ||
| Synonyms |
Suberohydroxamic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 14.5 nM | |||
| External Link | ||||
| ST-2986 | Investigative | [22] | ||
| Synonyms |
CHEMBL471041; SCHEMBL3444455; BDBM50278219
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 661 nM | |||
| External Link | ||||
| 9,9,9-Trifluoro-8-oxo-nonanoic acid phenylamide | Investigative | [21] | ||
| Synonyms |
9,9,9-Trifluoro-8-Oxo-N-Phenylnonanamide; CHEMBL113537; 2gh6; SCHEMBL2702892; KRCXZGYVOZSCSF-UHFFFAOYSA-N; BDBM50121062; DB07553
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 4500 nM | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid phenylamide | Investigative | [24] | ||
| Synonyms |
Thiol-SAHA (t-SAHA); CHEMBL325676; SCHEMBL14821761; BDBM152692
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 19 nM | |||
| External Link | ||||
| 6-benzenesulfinylhexanoic acid hydroxamide | Investigative | [25] | ||
| Synonyms |
6-(benzenesulfinyl)hexanoic acid hydroxyamide; 875737-03-4
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-Mercapto-ethyl)-N'-phenyl-succinamide | Investigative | [26] | ||
| Synonyms |
CHEMBL193959
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid biphenyl-4-ylamide | Investigative | [24] | ||
| Synonyms |
CHEMBL112311
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-phenylacetylamino-benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL356824; 656261-23-3; SCHEMBL675578; CTK1J6158; DTXSID40458440; ZINC13533297; AKOS030583151; Benzeneacetamide, N-[4-[(hydroxyamino)carbonyl]phenyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-(2-Bromo-acetylamino)-hexanoic acid phenylamide | Investigative | [24] | ||
| Synonyms |
CHEMBL344920; 651767-99-6; SCHEMBL3736839; CTK1J8444; DTXSID50432973; HWYLREOMBVUGJQ-UHFFFAOYSA-N; BDBM50222416; ZINC13587789; AKOS030603042; N-Phenyl-6-(bromoacetylamino)hexanamide; Hexanamide, 6-[(bromoacetyl)amino]-N-phenyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(phenylacetylamino-methyl)-benzamide | Investigative | [28] | ||
| Synonyms |
CHEMBL143674; SCHEMBL673760
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-4-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [29] | ||
| Synonyms |
CHEMBL126355; BDBM50222394
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Octanedioic acid hydroxyamide pyridin-4-ylamide | Investigative | [30] | ||
| Synonyms |
SCHEMBL8082656; CHEMBL165162; ZINC13472304
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(6-Mercapto-hexyl)-benzamide | Investigative | [24] | ||
| Synonyms |
CHEMBL112364; BDBM50223650
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Hydroxy-N-(5-hydroxycarbamoyl-pentyl)-benzamide | Investigative | [30] | ||
| Synonyms |
CHEMBL167455
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-((R)-2-phenyl-butyrylamino)-benzamide | Investigative | [27] | ||
| Synonyms |
SCHEMBL675474
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-benzenesulfonylhexanoic acid hydroxamide | Investigative | [25] | ||
| Synonyms |
CHEMBL203207
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 9-(Biphenyl-4-yloxy)-1,1,1-trifluoro-nonan-2-one | Investigative | [21] | ||
| Synonyms |
SCHEMBL7373122; CHEMBL116578
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Thioacetic acid S-(6-phenylcarbamoyl-hexyl) ester | Investigative | [24] | ||
| Synonyms |
CHEMBL111806; SCHEMBL14812153
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Butyrylamino-N-hydroxy-benzamide | Investigative | [28] | ||
| Synonyms |
CHEMBL142254; 656261-22-2; Benzamide, N-hydroxy-4-[(1-oxobutyl)amino]-; SCHEMBL675234; CTK1J6159; DTXSID90461262
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Naphthalen-2-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [29] | ||
| Synonyms |
CHEMBL127328
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid biphenyl-3-ylamide | Investigative | [24] | ||
| Synonyms |
CHEMBL320323
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione | Investigative | [31] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(2-phenyl-butyrylamino)-benzamide | Investigative | [27] | ||
| Synonyms |
SCHEMBL676079
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Dimethylamino-N-(6-mercapto-hexyl)-benzamide | Investigative | [24] | ||
| Synonyms |
CHEMBL324126
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid pyridin-3-ylamide | Investigative | [24] | ||
| Synonyms |
CHEMBL332246; Heptanamide, 7-mercapto-N-3-pyridinyl-; BDBM50223653
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-Phenoxy-hexane-1-thiol | Investigative | [24] | ||
| Synonyms |
CHEMBL109796; 6-phenoxyhexane-1-thiol; 1-Hexanethiol, 6-phenoxy-; SCHEMBL5679745; MolPort-020-180-823; BDBM50223652; AKOS018584222; MCULE-9521857089
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Benzoylamino-N-hydroxy-benzamide | Investigative | [27] | ||
| Synonyms |
SCHEMBL673678; CHEMBL191227
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Chloro-N-(5-hydroxycarbamoyl-pentyl)-benzamide | Investigative | [21] | ||
| Synonyms |
CHEMBL143734; NSC718168; AC1L8L82; SCHEMBL13039735; ZINC5579677; BDBM50082664; NSC-718168; NCI60_040737; 6-(4-Chlorobenzoylamino)hexanehydroxamic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-(Biphenyl-4-yloxy)-1,1,1-trifluoro-octan-2-one | Investigative | [29] | ||
| Synonyms |
CHEMBL112148; SCHEMBL7364383; BDBM50218532
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-3-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [29] | ||
| Synonyms |
CHEMBL127351; SCHEMBL7365180; HWZHDGRMABBYOV-UHFFFAOYSA-N; BDBM50222367; 7-((1,1'-biphenyl)-3-yloxy)-1-(1 ,3-oxazol-2-yl)-1-heptanone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-Mercapto-hexanoic acid phenylamide | Investigative | [24] | ||
| Synonyms |
CHEMBL109654; Hexanamide, 6-mercapto-N-phenyl-; SCHEMBL14254925; BDBM50027600
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 67 nM | |||
| External Link | ||||
| Cyclostellettamine derivative | Investigative | [32] | ||
| Synonyms |
CHEMBL88332
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(5-Hydroxycarbamoyl-pentyl)-4-nitro-benzamide | Investigative | [21] | ||
| Synonyms |
CHEMBL139999; SCHEMBL1232700; BDBM50082661; ZINC13472309
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-Mercapto-pentanoic acid phenylamide | Investigative | [24] | ||
| Synonyms |
N-Phenyl-5-mercaptovaleramide; CHEMBL114344; Pentanamide, 5-mercapto-N-phenyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Octanedioic acid hydroxyamide pyridin-2-ylamide | Investigative | [30] | ||
| Synonyms |
SCHEMBL8090513; CHEMBL164872; ZINC13472303
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-Mercapto-ethyl)-N'-phenyl-oxalamide | Investigative | [26] | ||
| Synonyms |
CHEMBL193979
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(methylsulfonylthio)ethyl 2-propylpentanoate | Investigative | [31] | ||
| Synonyms |
CHEMBL271677; SCHEMBL4156413
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (E)-8-Biphenyl-4-yl-1-oxazol-2-yl-oct-7-en-1-one | Investigative | [29] | ||
| Synonyms |
CHEMBL126465; SCHEMBL7368197; SCHEMBL7368201
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-((S)-2-phenyl-butyrylamino)-benzamide | Investigative | [27] | ||
| Synonyms |
SCHEMBL676080
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(3-phenyl-propionylamino)-benzamide | Investigative | [27] | ||
| Synonyms |
N-hydroxy-4-(3-phenylpropanamido)benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(5-phenyl-pentanoylamino)-benzamide | Investigative | [27] | ||
| Synonyms |
SCHEMBL7311087
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-Oxo-8-phenyl-octanoic acid | Investigative | [30] | ||
| Synonyms |
8-Oxo-8-phenyloctanoic acid; 7-Benzoylheptanoic acid; 24314-23-6; Benzeneoctanoic acid, h-oxo-; 7-BENZOYL HEPTANOIC ACID; AC1L6TSB; SCHEMBL3381106; 8-keto-8-phenyl-caprylic acid; CHEMBL162423; 8-Oxo-8-phenyloctanoic acid #; CTK4F3363; DTXSID40305602; UMCSRRHQLAVYRS-UHFFFAOYSA-N; ZINC2168376; 7009f; NSC171230; AKOS016022495; NSC-171230; MCULE-7202530747; ACM24314236; ST50825837
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(4-phenyl-butyrylamino)-benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL143336; 656261-24-4; SCHEMBL674421; CTK1J6157; DTXSID30433908; ZINC13533300; AKOS030583673; n-hydroxy-4-(4-phenylbutyryl-amino)benzamide; Benzenebutanamide, N-[4-[(hydroxyamino)carbonyl]phenyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-phenylsulfanylhexanoic acid hydroxamide | Investigative | [25] | ||
| Synonyms |
Hexanamide, N-hydroxy-6-(phenylthio)-; CHEMBL203028; SCHEMBL7317658
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ST-2987 | Investigative | [22] | ||
| Synonyms |
CHEMBL471042; SCHEMBL3444989; SCHEMBL3444984; BDBM50278220
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 124 nM | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid quinolin-3-ylamide | Investigative | [24] | ||
| Synonyms |
CHEMBL112234
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-(4-Chloro-phenyl)-pentanoic acid hydroxyamide | Investigative | [33] | ||
| Synonyms |
CHEMBL84288
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-Mercapto-octanoic acid phenylamide | Investigative | [24] | ||
| Synonyms |
8-mercapto-N-phenyloctanamide; CHEMBL326433; ZINC13609343
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 70 nM | |||
| External Link | ||||
| N-(6-Hydroxycarbamoyl-hexyl)-benzamide | Investigative | [30] | ||
| Synonyms |
CHEMBL57107; 174664-71-2; SCHEMBL573254; CTK0A7470; DTXSID00433435; BDBM50220823; ZINC13490043; 7-(Benzoylamino)heptanehydroxamic acid; AKOS030580013; Benzamide, N-[7-(hydroxyamino)-7-oxoheptyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-4-yloxy)-1,1,1-trifluoro-heptan-2-one | Investigative | [21] | ||
| Synonyms |
CHEMBL326529; SCHEMBL7365237; BDBM50217957
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid benzothiazol-2-ylamide | Investigative | [24] | ||
| Synonyms |
CHEMBL178779
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(pentanoylamino-methyl)-benzamide | Investigative | [28] | ||
| Synonyms |
CHEMBL143102
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PSAMMAPLIN A | Investigative | [21] | ||
| Synonyms |
110659-91-1; Bisprasin; NSC614495; AC1O46WI; SCHEMBL364511; ZINC150352860; NSC-614495; B723735K022; J-002461; Benzenepropanamide, N,N'-(dithiodi-2,1-ethanediyl)bis(3-bromo-4-hydroxy-alpha-(hydroxyimino)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
References