m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03136
|
[1] | |||
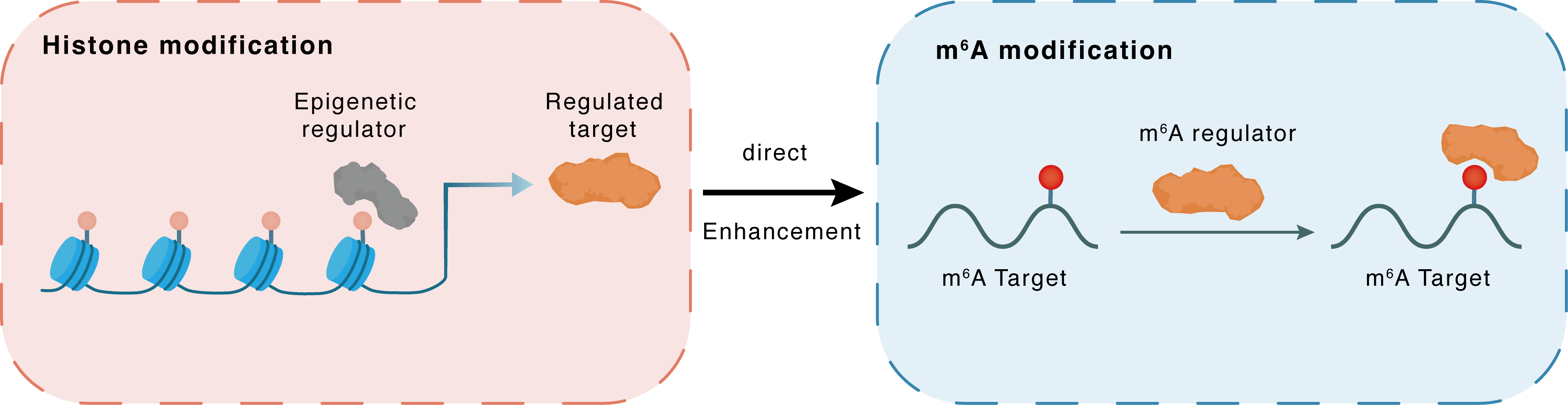 Histone modification
H3K18la
EP300
YTHDC1
Direct
Enhancement
m6A modification
NEAT1
NEAT1
YTHDC1
Histone modification
H3K18la
EP300
YTHDC1
Direct
Enhancement
m6A modification
NEAT1
NEAT1
YTHDC1
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing protein 1 (YTHDC1) | READER | |||
| m6A Target | Nuclear paraspeckle assembly transcript 1 (NEAT1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone acetyltransferase p300 (P300) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 18 lactylation (H3K18la) | View Details | |||
| Downstream Gene | YTHDC1 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | Histone H3 lysine 18 lactylation (H3K18la) in HCC induces increased expression of YTHDC1, increasing the stability of m6A-modified Nuclear paraspeckle assembly transcript 1 (NEAT1). The histone acetyltransferase p300 is then recruited by NEAT1 and activates SCD by increasing the level of histone acetylation at the SCD promoter, thus facilitating HCC progression via hepatocellular lipid metabolism remodeling. Taken together, these discoveries suggest a close link between the epigenetic machinery and lipid metabolic abnormalities, which promotes cancer progression. | ||||
| Responsed Disease | Liver cancer | ICD-11: 2C12 | |||
In-vitro Model |
Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Histone acetyltransferase p300 (P300) | 2 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| CCS1477 | Phase 1/2 | [2] | ||
| Synonyms |
CCS-1477; CBP-IN-1; 2222941-37-7; (S)-1-(3,4-Difluorophenyl)-6-(5-(3,5-dimethylisoxazol-4-yl)-1-((1r,4S)-4-methoxycyclohexyl)-1H-benzo[d]imidazol-2-yl)piperidin-2-one; SCHEMBL20094038; SCHEMBL21515367; SCHEMBL22134021; EX-A3687; NSC818619; NSC-818619; HY-111784; CS-0091862; (S)-1-(3,4-Difluorophenyl)-6-(5-(3,5-dimethylisoxazol-4-yl)-1-(trans-4-methoxycyclohexyl)-1H-benzo[d]imidazol-2-yl)piperidin-2-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| FT-7051 | Phase 1 | [3] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2C12: Liver cancer | 49 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| 90Y-loaded resin microspheres | Approved | [4] | ||
| External Link | ||||
| Thymalfasin | Phase 2 | [5] | ||
| Synonyms |
Zadaxin; 62304-98-7; Thymosin alpha1; Thymosin alpha 1; Thymosin alpha1 (ox); Thymosin alpha1 (human); Thymalfasin [USAN:INN]; UNII-W0B22ISQ1C; Thymosin-alpha-1; 69440-99-9; Thymosin alpha1 (cattle); C129H215N33O55; W0B22ISQ1C; Zadaxin (TN); Thymalfasin alfa 1
Click to Show/Hide
|
|||
| External Link | ||||
| Ferumoxides | Approved | [6] | ||
| Synonyms |
AMI-25; 119683-68-0; Feridex; Feridex IV; Superparamagnetic iron oxide; UNII-G6N3J05W84; Ferumoxides [USAN:USP:BAN]; CCRIS 6722; HSDB 8072; AC1O5DID; G6N3J05W84; iron(2+); iron(3+); Iron oxide crystal is inverse spinel (X-ray data); Fe(II) and Fe(III) are present (Mossbauer Spectroscopy; Physical form is a colloidal particle of nonstoichiometric
Click to Show/Hide
|
|||
| External Link | ||||
| DTI-015 | Approved | [7] | ||
| Synonyms |
Carmustine; 154-93-8; 1,3-Bis(2-chloroethyl)-1-nitrosourea; BCNU; Carmustin; Nitrumon; Carmubris; Gliadel; BiCNU; Bi CNU; Carmustinum; Bischlorethylnitrosurea; Bischlorethylnitrosourea; Carmustina; Becenun; Becenum; Bischloroethyl nitrosourea; N,N'-BIS(2-CHLOROETHYL)-N-NITROSOUREA; Bis(2-chloroethyl)nitrosourea; Urea, N,N'-bis(2-chloroethyl)-N-nitroso-; Gliadel Wafer; FDA 0345; Bischloroethylnitrosourea; SRI 1720; 1,3-Bis(2-chloroethyl)nitrosourea; BiCNU (TN); Carmustinum [INN-Latin]; Carmustina [INN-Spanish]; DTI 015; NCI-C04773; SK; Injectable carmustine, Direct Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| Nofazinlimab | Phase 3 | [8] | ||
| Synonyms |
CS1003; EQ176
Click to Show/Hide
|
|||
| External Link | ||||
| PV-10 | Phase 3 | [9] | ||
| Synonyms |
632-69-9; Rose bengal sodium; Rose bengal disodium salt; R105 sodium; Rose-bengal (131 I) natrium; Food Red No 105, sodium salt; EINECS 211-183-3; Food Red Color No 105, sodium salt; Sel disodique de rose bengale iodee (131 I); Rose bengale (131 I) sodique [INN-French]; Rosa bengala sodica (131 I) [INN-Spanish]; Roseum bengalense (131 I) natricum [INN-Latin]; 2,4,5,7-Tetraido(m,p,o',m')tetrachlorofluorescein, disodium salt; Fluorescein, 4,5,6,7-tetrachloro-2',4',5',7'-tetraiodo-, disodium salt; Disodium
Click to Show/Hide
|
|||
| External Link | ||||
| Brivanib | Phase 3 | [10] | ||
| Synonyms |
649735-46-6; BMS-540215; Brivanib (BMS-540215); BMS 540215; UNII-DDU33B674I; Brivanib [USAN]; BMS540215; DDU33B674I; CHEMBL377300; (2R)-1-[4-(4-Fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy]propanol; Brivanib (USAN); (2R)-1-[4-[(4-FLUORO-2-METHYL-1H-INDOL-5-YL)OXY]-5-METHYL-PYRROLO[2,1-F][1,2,4]TRIAZIN-6-YL]OXYPROPAN-2-OL; (2R)-1-({4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yl}oxy)propan-2-ol
Click to Show/Hide
|
|||
| External Link | ||||
| JX-594 | Phase 3 | [11] | ||
| Synonyms |
Pexastimogene devacirepvec
Click to Show/Hide
|
|||
| External Link | ||||
| ABH001 | Phase 3 | [12] | ||
| External Link | ||||
| MTC-DOX | Phase 2/3 | [13] | ||
| Synonyms |
MTC-doxorubicin
Click to Show/Hide
|
|||
| External Link | ||||
| KD018 | Phase 2 | [14] | ||
| External Link | ||||
| Doxorubicin-eluting beads | Phase 2 | [15] | ||
| Synonyms |
DC Bead (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| 32-P BioSilicon | Phase 2 | [16] | ||
| Synonyms |
BrachySil
Click to Show/Hide
|
|||
| External Link | ||||
| Cixutumumab | Phase 2 | [17] | ||
| Synonyms |
LY3012217
Click to Show/Hide
|
|||
| External Link | ||||
| [131I]-Metuximab | Phase 2 | [18] | ||
| External Link | ||||
| Darinaparsin | Phase 2 | [19] | ||
| Synonyms |
ZIO-101
Click to Show/Hide
|
|||
| External Link | ||||
| Tigatuzumab | Phase 2 | [20] | ||
| External Link | ||||
| CMC-001 | Phase 2 | [21] | ||
| Synonyms |
Manganese-based MRI contrast agent (liver tumor imaging), CMC Contrast
Click to Show/Hide
|
|||
| External Link | ||||
| OBP-301 | Phase 1/2 | [22] | ||
| External Link | ||||
| MBO7133 | Phase 1/2 | [23] | ||
| External Link | ||||
| INCB62079 | Phase 1/2 | [9] | ||
| External Link | ||||
| NV-1020 | Phase 1/2 | [24] | ||
| External Link | ||||
| DCVax-Liver | Phase 1/2 | [25] | ||
| Synonyms |
Dendritic cell-based immunotherapy (liver cancer), Northwest Biotherapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| SRF388 | Phase 1 | [26] | ||
| External Link | ||||
| ET140202 | Phase 1 | [27] | ||
| External Link | ||||
| ADP-A2AFP | Phase 1 | [28] | ||
| External Link | ||||
| SM04755 | Phase 1 | [29] | ||
| External Link | ||||
| Anti-CEA CAR-T therapy | Phase 1 | [9] | ||
| External Link | ||||
| PI-166 | Phase 1 | [30] | ||
| External Link | ||||
| CRS-100 | Phase 1 | [31] | ||
| External Link | ||||
| Autologous ET1402L1-CART cells | Phase 1 | [32] | ||
| External Link | ||||
| Anti-CD133-CAR vector-transduced T cells | Phase 1 | [33] | ||
| External Link | ||||
| MRX34 | Phase 1 | [34] | ||
| External Link | ||||
| ALN-VSP | Phase 1 | [35] | ||
| External Link | ||||
| EPCAM-targeted CAR-T cells | Clinical trial | [36] | ||
| External Link | ||||
| ADI | Discontinued in Phase 3 | [37] | ||
| Synonyms |
Arginine deiminase
Click to Show/Hide
|
|||
| External Link | ||||
| GN-1140 | Discontinued in Phase 2 | [38] | ||
| External Link | ||||
| OGT-719 | Discontinued in Phase 2 | [39] | ||
| Synonyms |
OGS-719
Click to Show/Hide
|
|||
| External Link | ||||
| AFP-Scan | Discontinued in Phase 2 | [40] | ||
| External Link | ||||
| SR1078 | Preclinical | [41] | ||
| Synonyms |
1246525-60-9; SR 1078; SR-1078; N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-4-(trifluoromethyl)benzamide; CHEMBL3094387; N-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl)-4-(trifluoromethyl)benzamide; N-[4-[2,2,2-Trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-4-(trifluoromethyl)benzamide; SCHEMBL4880524; C17H10F9NO2; DTXSID30591895; BCP09203; EX-A2215; 4063AH; BDBM50444350; s5775; ZINC98052696; AKOS024458390; CS-1045; NCGC00379222-02; AK547149; AS-55921; HY-14422; W-5797; SR-03000001078; SR-03000001078-1; SR-03000001078-2
Click to Show/Hide
|
|||
| External Link | ||||
| Occlusin | Preclinical | [42] | ||
| Synonyms |
Occlusin 50 Injection; Occlusin 500 injection
Click to Show/Hide
|
|||
| External Link | ||||
| HRC-201 | Terminated | [43] | ||
| Synonyms |
Hemoglobin-imaging conjugate (HepSelect), Hemosol
Click to Show/Hide
|
|||
| External Link | ||||
| 1,2,3,4,5,6-hexabromocyclohexane | Investigative | [44] | ||
| Synonyms |
1837-91-8; Benzene hexabromide; Cyclohexane, 1,2,3,4,5,6-hexabromo-; Hexabromocyclohexane; JAK2 Inhibitor II; ACMC-1BQJT; SCHEMBL459442; trans-alpha-Benzene hexabromide; CHEMBL444236; DTXSID4052687; CHEBI:93940; NSC7908; HMS3268H22; HMS3413C10; HMS3677C10; NSC-7908; ZINC1586309; ANW-23174; MFCD00059127; s5902; Cyclohexane,2,3,4,5,6-hexabromo-; AKOS015836040; 1,2,3,4,5,6-Hexabromo-cyclohexane; 1,2,3,4,5,6-Hexabromocyclohexane #; NCGC00092358-01; NCGC00092358-02; 1,2,3,4,5,6-hexakis(bromanyl)cyclohexane; A4510; FT-0633875; JAK2 Inhibitor II - CAS 1837-91-8; 1,2,3,4,5,6-Hexabromocyclohexane;NSC7908; A812818; 1,2,3,4,5,6-Hexabrom-cyclohexan (I(2)-Form); J-011778; 1,2,3,4,5,6-Hexabromocyclohexane, >=98% (HPLC); BRD-K06817181-001-01-5; Q27165694
Click to Show/Hide
|
|||
| External Link | ||||
| STP-322 | Investigative | [45] | ||
| Synonyms |
Multi-targeted siRNA therapeutic cocktail (nanoparticle, liver tumor), Sirnaomics
Click to Show/Hide
|
|||
| External Link | ||||
| AMB-8LK | Investigative | [45] | ||
| Synonyms |
Cancer therapy (monoclonal antibody), MAT Biopharma; Y90 anti-ferritin monoclonal antibody (cancer), MAT Biopharma; Yttrium-90 anti-ferritin mAb (cancer), MAT Biopharma; Yttrium-90 anti-ferritin mAb (Hodgkin's disease/pancreatic/liver cancer), MAT Biopharma; 90Y-AMB8LK mAb (cancer), MAT Biopharma; 90Y-AMB8LK monoclonal antibody (cancer), MAT Biopharma; 90Y-labelled anti-ferritin monoclonal antibody (cancer), MAT Biopharma
Click to Show/Hide
|
|||
| External Link | ||||
| MiR-34a mimics | Investigative | [45] | ||
| Synonyms |
MiR-34a mimics (liver cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| P53 fusion protein | Investigative | [45] | ||
| Synonyms |
P53 fusion protein (pancreatic/liver cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| OP-05 | Investigative | [45] | ||
| Synonyms |
OP-05 program (prodrug, liver tumor); OP-05 program (prodrug, liver tumor), Onco-Pharmakon
Click to Show/Hide
|
|||
| External Link | ||||
| GR-DD1 | Investigative | [45] | ||
| Synonyms |
Cytotoxin (hepatic metastasis), ERYtech
Click to Show/Hide
|
|||
| External Link | ||||
References