m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT02228
|
[1], [2] | |||
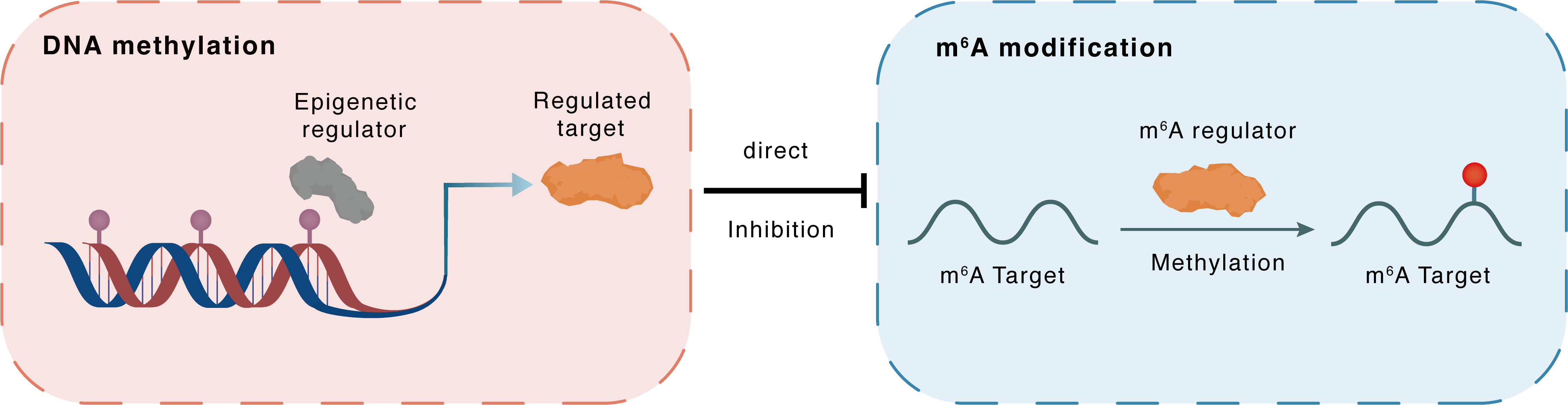 DNA methylation
DNMT3A
METTL14
Direct
Inhibition
m6A modification
CXCR4
CXCR4
METTL14
Methylation
DNA methylation
DNMT3A
METTL14
Direct
Inhibition
m6A modification
CXCR4
CXCR4
METTL14
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 14 (METTL14) | WRITER | |||
| m6A Target | C-X-C chemokine receptor type 4 (CXCR4) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Epigenetic Regulator | Cysteine methyltransferase DNMT3A (DNMT3A) | WRITER | View Details | ||
| Regulated Target | Methyltransferase-like protein 14 (METTL14) | View Details | |||
| Crosstalk Relationship | DNA methylation → m6A | Inhibition | |||
| Crosstalk Mechanism | DNA methylation directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | lncRNA UCA1 recruited DNA methyltransferase (DNMT1, DNMT3A, and DNMT3B) to the METTL14 promoter region to inhibit METTL14 expression in breast cancer. LNC942-METTL14-C-X-C chemokine receptor type 4 (CXCR4)/CYP1B1 signaling axis, which provides new targets and crosstalk m6A epigenetic modification mechanism for breast cancer prevention and treatment. | ||||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | |||
| Pathway Response | Apoptosis | hsa04210 | |||
| Cell Process | Cell apoptosis | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| C-X-C chemokine receptor type 4 (CXCR4) | 61 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Plerixafor | Approved | [3] | ||
| Synonyms |
Mozobil; AMD3100; Amd 3100; JM 2987; JM 3100; JM3100; SDZ SID 791; SID791; AMD-3100; Bicyclam JM-2987; JM-3100; Mozobil (TN); GNA & AMD-3100; HHA & AMD-3100; Plerixafor (INN/USAN); 1,1'-[1,4-Phenylenebis(methylene)]bis[1,4,8,11-tetraazacyclotetradecane]; 1,1'-[1,4-Phenylenebis(methylene)]bis[1,4,8,11-tetraazacyclotetradecane] & Galanthus nivalis agglutinin (GNA); 1,1'-[1,4-Phenylenebis(methylene)]bis[1,4,8,11-tetraazacyclotetradecane] & Hippeastrum hybrid agglutinin(HHA); 1,1'-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-tetraaza-cyclotetradecane}; 1-[[4-(1,4,8,11-tetrazacyclotetradec-1-ylmethyl)phenyl]methyl]-1,4,8,11-tetrazacyclotetradecane
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | IC50 = 0.057 ug.mL-1 | |||
| External Link | ||||
| Motixafortide | Approved | [4] | ||
| MOA | Antagonist | |||
| External Link | ||||
| Ulocuplumab | Phase 3 | [5] | ||
| MOA | Antagonist | |||
| External Link | ||||
| Balixafortide | Phase 3 | [6] | ||
| Synonyms |
1051366-32-5; Balixafortide [INN]; UNII-PRC974M49B; PRC974M49B; Ala-cys-ser-ala-pro-arg-tyr-cys-tyr-gln-lys-pro-pro-tyr-his cyclic (2->9)-disulfide; Cyclo(L-alanyl-L-cysteinyl-L-seryl-L-alanyl-D-prolyl-(2S)-2,4-diaminobutanoyl-L-arginyl-L-tyrosyl-L-cysteinyl-L-tyrosyl-L-glutaminyl-L-lysyl-D-prolyl-L-prolyl-L-tyrosyl-L-histidyl), cyclic (2->9)-disulfide
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| AMD-070 | Phase 3 | [7] | ||
| Synonyms |
AMD 070; AMD070; AMD11070; S14-0353; N-(1H-benzoimidazol-2-ylmethyl)-N-(5,6,7,8-tetrahydroquinolin-8-yl)butane-1,4-diamine; N'-(1H-Benzo[d]imidazol-2-ylmethyl)-N'-(5,6,7,8-tetrahydroquinolin-8-yl)butane-1,4-diamine Trihydrobromide Dihydrate
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | IC50 = 13 nM | |||
| External Link | ||||
| TG-0054 | Phase 2 | [8] | ||
| Synonyms |
CXCR4 binding inhibitor/cell mobilizer (iv, stem cell transplant), TaiGen Biotechnology
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| POL-6326 | Phase 2 | [9] | ||
| Synonyms |
CXCR4 antagonists, Polyphor; POL-2438; POL-3026; Epitope mimetics (HIV fusion), Polyphor; CXCR4 antagonists (cancer/HIV), Polyphor
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| CTCE-9908 | Phase 1/2 | [10] | ||
| Synonyms |
Anticancer therapy, Chemokine Therapeutics; CTCE-9908/0019
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| USL311 | Phase 1/2 | [11] | ||
| Synonyms |
Usl-311; UNII-2BTG5MX2Q2; 2BTG5MX2Q2; SCHEMBL15347153; 2-Pyridinecarboxamide, 6-(hexahydro-4-(1-(1-methylethyl)-4-piperidinyl)-1H-1,4-diazepin-1-yl)-N-4-pyridinyl-; 1373268-67-7
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| ALX-0651 | Phase 1 | [12] | ||
| Synonyms |
Anti-CXCR4 nanobodies (cancer), Ablynx
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CTCE-0214 | Phase 1 | [13] | ||
| Synonyms |
CTCE-0013; CTCE-0021; CXCR4 agonists, Chemokine Therapeutics; Stem cell transplant therapy, Chemokine Therapeutics
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| MSX-122 | Phase 1 | [14] | ||
| MOA | Antagonist | |||
| External Link | ||||
| PF-06747143 | Phase 1 | [5] | ||
| MOA | Antagonist | |||
| External Link | ||||
| BMS-936564 | Phase 1 | [15] | ||
| MOA | Modulator | |||
| External Link | ||||
| LY2624587 | Phase 1 | [16] | ||
| MOA | Antagonist | |||
| External Link | ||||
| GMI-1359 | Phase 1 | [17] | ||
| MOA | Antagonist | |||
| External Link | ||||
| Garnocestim | Discontinued in Phase 1 | [18] | ||
| Synonyms |
SB-251353
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| SURADISTA | Discontinued in Phase 1 | [19] | ||
| Synonyms |
PNU-145156E; FCE-26644 (formerly); PNU-151484 (Na salt); 7,7'-[Carbonylbis[imino(1-methyl-1H-pyrrole-4,2-diyl)carbonylimino(1-methyl-1H-pyrrole-4,2-diyl)carbonylimino]]bis-1,3-naphthalenedisulfonic acid tetrapotassium salt; 7,7-Ureylene-bis(1-methyl-4,2-pyrrolecarboxamido)bis(1-methyl-4,2-pyrrolecarboxamido)bis(1,3-naphthalenedisulfonic acid) tetrapotassium salt
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| MAb173 | Preclinical | [20] | ||
| MOA | Antagonist | |||
| External Link | ||||
| KRH-2731 | Terminated | [21] | ||
| MOA | Binder | |||
| External Link | ||||
| Cyclo(-D-Tyr-L-Arg-L-MeArg-L-Nal-Gly-) | Investigative | [22] | ||
| Synonyms |
CHEMBL436536
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 99 nM | |||
| External Link | ||||
| Cyclo(-D-MeTyr-D-Arg-L-Arg-L-Nal-Gly-) | Investigative | [22] | ||
| Synonyms |
CHEMBL375993
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 157 nM | |||
| External Link | ||||
| Cyclo(-D-Tyr-D-MeArg-L-Arg-L-Nal-Gly-) | Investigative | [22] | ||
| Synonyms |
CHEMBL218806
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| GSK-812397 | Investigative | [23] | ||
| Synonyms |
CXCR4 receptor antagonists (HIV-1 infection); CXCR4 receptor antagonists (HIV-1 infection), GlaxoSmithKline
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | IC50 = 0.87 nM | |||
| External Link | ||||
| AT-009 | Investigative | [23] | ||
| MOA | Antagonist | |||
| External Link | ||||
| Cyclo(-D-Tyr-D-Arg-L-Arg-L-MeNal-Gly-) | Investigative | [22] | ||
| Synonyms |
CHEMBL426169
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 563 nM | |||
| External Link | ||||
| Cyclo(-D-Tyr-L-Arg-L-Arg-L-Nal-L-Ala-) | Investigative | [22] | ||
| Synonyms |
CHEMBL387120
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 170 nM | |||
| External Link | ||||
| Cyclo(-D-Tyr-L-MeArg-L-Arg-L-Nal-Gly-) | Investigative | [22] | ||
| Synonyms |
CHEMBL374421
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 23 nM | |||
| External Link | ||||
| Cyclo(-D-Tyr-Arg-Arg-Nal-Gly-) | Investigative | [24] | ||
| Synonyms |
Fc-131; FC131; CHEMBL436283; FC 131; 606968-52-9; CHEMBL2180076; AC1NQNK6; SCHEMBL15987252; ZINC3925712; Cyclo(-Nal-Gly-D-Tyr-Arg-Arg-); BDBM50399002; BDBM50166106; KB-272560; B7647; N-{3-[(2S,5S,8S,14R)-5-(3-Guanidino-propyl)-14-(4-hydroxy-benzyl)-8-naphthalen-2-ylmethyl-3,6,9,12,15-pentaoxo-1,4,7,10,13pentaaza-cyclopentadec-2-yl]-propyl}-guanidine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| Cyclo(-D-Tyr-D-Arg-L-Arg-L-Nal-beta-Ala-) | Investigative | [22] | ||
| Synonyms |
CHEMBL219135
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 350 nM | |||
| External Link | ||||
| KUR-CXCR4 | Investigative | [23] | ||
| MOA | Modulator | |||
| External Link | ||||
| isothiourea-1t | Investigative | [25] | ||
| Synonyms |
IT1t
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | IC50 = 8 nM | |||
| External Link | ||||
| Cyclo(-D-Tyr-D-Arg-L-Arg-L-Nal-Sar-) | Investigative | [22] | ||
| Synonyms |
CHEMBL374862
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 256 nM | |||
| External Link | ||||
| CX-02 | Investigative | [23] | ||
| Synonyms |
CX-05; CXCR4 monoclonal antibodies (cancer); CXCR4 monoclonal antibodies (cancer), Northwest Biotherapeutics; CXCR4 therapeutics (cancer), Northwest Biotherapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| isothiourea-1a | Investigative | [25] | ||
| Synonyms |
IT1a
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | IC50 = 11 nM | |||
| External Link | ||||
| ND-401 | Investigative | [23] | ||
| Synonyms |
ND-4019; CCR5 and CXCR4 inhibitors (HIV infection), NeED Pharmaceuticals
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CXCR4 gene disrupted T cells | Investigative | [23] | ||
| Synonyms |
CXCR4 gene disrupted T cells (HIV infection)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| T134 | Investigative | [26] | ||
| Synonyms |
GTPL852
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Cyclo(-D-Tyr-L-Arg-L-Arg-L-Ala-Sar-) | Investigative | [22] | ||
| Synonyms |
CHEMBL219075
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 167 nM | |||
| External Link | ||||
| Cyclo(-D-Tyr-D-Arg-L-MeArg-L-Nal-Gly-) | Investigative | [22] | ||
| Synonyms |
CHEMBL376219
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 21 nM | |||
| External Link | ||||
| ATI-2341 | Investigative | [23] | ||
| Synonyms |
ATI-2346; ATI-2756; ATI-2766; CXCR4 agonists (cancer/bone marrow transplantation), Ascent; Pepducins (cancer/bone marrow transplantation), Ascent
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Cyclo(-D-Tyr-L-Arg-L-Arg-L-MeNal-Gly-) | Investigative | [22] | ||
| Synonyms |
CHEMBL375850
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 250 nM | |||
| External Link | ||||
| Cyclo(-D-Tyr-D-Arg-L-Arg-L-Nal-L-Ala-) | Investigative | [22] | ||
| Synonyms |
CHEMBL376811
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 92 nM | |||
| External Link | ||||
| Cyclo(-D-Tyr-D-Ala-L-Arg-L-Nal-Gly-) | Investigative | [22] | ||
| Synonyms |
CHEMBL384429
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 230 nM | |||
| External Link | ||||
| T140 | Investigative | [26] | ||
| Synonyms |
[L-3-(2-naphthyl)-alanine3]-T134
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Cyclo(-D-Tyr-D-Arg-L-Arg-L-Nal-D-Ala-) | Investigative | [22] | ||
| Synonyms |
CHEMBL373636
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 11 nM | |||
| External Link | ||||
| Cyclo(-D-Ala-D-Arg-L-Arg-L-Nal-Gly-) | Investigative | [22] | ||
| Synonyms |
CHEMBL373440
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 130 nM | |||
| External Link | ||||
| CTCE-0324 | Investigative | [23] | ||
| Synonyms |
Vascular disease therapeutic, Chemokine Therapeutics
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Cyclo(-D-Tyr-L-Ala-L-Arg-L-Nal-Gly-) | Investigative | [22] | ||
| Synonyms |
CHEMBL374108
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 63 nM | |||
| External Link | ||||
| Cyclo(-D-Tyr-L-Arg-L-Arg-L-Nal-beta-Ala-) | Investigative | [22] | ||
| Synonyms |
CHEMBL375991
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 47 nM | |||
| External Link | ||||
| Cyclo(-D-Tyr-L-Arg-L-Arg-L-Nal-Gly-) | Investigative | [22] | ||
| Synonyms |
CHEMBL219474
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| LP-0067 | Investigative | [23] | ||
| Synonyms |
CXCR4 antagonists (autoimmune disease); CXCR4 antagonists (autoimmune disease), Leo Pharma
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| NB-325 | Investigative | [23] | ||
| Synonyms |
PEHMB; Polyethylene hexamethylene biguanide
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Cyclo(-D-MeTyr-L-Arg-L-Arg-L-Nal-Gly-) | Investigative | [22] | ||
| Synonyms |
CHEMBL219096
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 128 nM | |||
| External Link | ||||
| Cyclo(-D-Tyr-L-Arg-L-Arg-L-Nal-D-Ala-) | Investigative | [22] | ||
| Synonyms |
CHEMBL375990
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 11 nM | |||
| External Link | ||||
| Cyclo(-D-Tyr-D-Arg-L-Arg-L-Nal-L-Pic-) | Investigative | [22] | ||
| Synonyms |
CHEMBL373637
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 640 nM | |||
| External Link | ||||
| T22 | Investigative | [26] | ||
| Synonyms |
[Tyr5,12,Lys7]-polyphemusin II
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| TN-14003 | Investigative | [27] | ||
| Synonyms |
UNII-1TW3FT746I; 1TW3FT746I; TN14003; BDBM194584; US9205085, MSX-207; H-Arg-Arg-Nal-Cys-Tyr-Cit-Lys-DLys-Pro-Tyr-Arg-Cit-Cys-Arg-NH2; L-Argininamide, L-arginyl-L-arginyl-3-(2-naphthalenyl)-L-alanyl-L-cysteinyl-L-tyrosyl-N5-(aminocarbonyl)-L-ornithyl-L-lysyl-D-lysyl-L-prolyl-L-tyrosyl-L-arginyl-N5-(aminocarbonyl)-L-ornithyl-L-cysteinyl-, cyclic (4->13)-disulfide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo(-D-Tyr-D-Arg-L-Arg-L-Nal-Gly-) | Investigative | [22] | ||
| Synonyms |
CHEMBL219339
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8 nM | |||
| External Link | ||||
| CXCL8 | Investigative | [28] | ||
| Synonyms |
Interleukin-8; CHEMBL411250; IL-8
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| viral macrophage inflammatory protein-II | Investigative | [29] | ||
| Synonyms |
CFLTKRGRQVC; vMIP-II; AC1LAF0N; GTPL768; vMIP-II (RESIDUE 41-51, CYCLIC); (4R,7S,10S,13S,19S,22S,25S,28S,31S,34R)-34-amino-22-(4-aminobutyl)-10-(3-amino-3-oxopropyl)-31-benzyl-13,19-bis[3-(diaminomethylideneamino)propyl]-25-[(1R)-1-hydroxyethyl]-28-(2-methylpropyl)-6,9,12,15,18,21,24,27,30,33-decaoxo-7-propan-2-yl-1,2-dithia-5,8,11,14,17,20,23,26,29,32-decazacyclopentatriacontane-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Cysteine methyltransferase DNMT3A (DNMT3A) | 8 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PMID27376512-Compound-Figure3CN | Patented | [30] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 1100 nM | |||
| External Link | ||||
| PMID27376512-Compound-Figure3CG | Patented | [30] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 2400 nM | |||
| External Link | ||||
| PMID27376512-Compound-Figure3CM | Patented | [30] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 1100 nM | |||
| External Link | ||||
| PMID27376512-Compound-Figure2aExample1 | Patented | [30] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3000 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-424 | Patented | [30] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1940 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-427 | Patented | [30] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 295 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-422 | Patented | [30] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1430 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-423 | Patented | [30] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 363 nM | |||
| External Link | ||||
| 2C60: Breast cancer | 2 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Entrectinib | Approved | [31] | ||
| Synonyms |
1108743-60-7; RXDX-101; UNII-L5ORF0AN1I; Entrectinib (RXDX-101); L5ORF0AN1I; Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-; Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-; Entrectinib [USAN:INN]; YMX; Kinome_2659; Entrectinib(rxdx-101); Entrectinib (USAN/INN); SCHEMBL3512601; GTPL8290; CHEMBL1983268; KS-00000TSK
Click to Show/Hide
|
|||
| External Link | ||||
| Everolimus | Approved | [32] | ||
| External Link | ||||
References