m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT02218
|
[1], [2] | |||
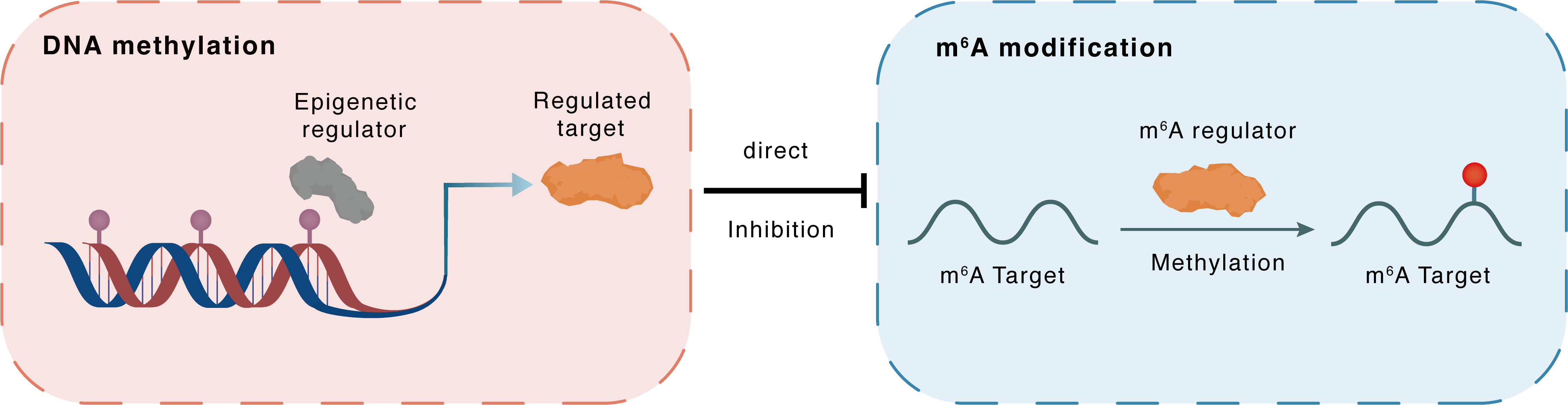 DNA methylation
DNMT3B
METTL14
Direct
Inhibition
m6A modification
GSK3B
GSK3B
METTL14
Methylation
DNA methylation
DNMT3B
METTL14
Direct
Inhibition
m6A modification
GSK3B
GSK3B
METTL14
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 14 (METTL14) | WRITER | |||
| m6A Target | Glycogen synthase kinase-3 beta (GSK3Beta/GSK3B) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 3B (DNMT3B) | WRITER | View Details | ||
| Regulated Target | Methyltransferase-like protein 14 (METTL14) | View Details | |||
| Crosstalk Relationship | DNA methylation → m6A | Inhibition | |||
| Crosstalk Mechanism | DNA methylation directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | lncRNA UCA1 recruited DNA methyltransferase (DNMT1, DNMT3A, and DNMT3B) to the METTL14 promoter region to inhibit METTL14 expression in breast cancer. m6A-hypomethylation regulated FGFR4 phosphorylates Glycogen synthase kinase-3 beta (GSK3Beta/GSK3B) and activates beta-catenin/TCF4-SLC7A11/FPN1 signaling to drive anti-HER2 resistance. Knockdown of METTL14 significantly increased the expression level of FGFR4 in HER2-positive breast cancer cells. FGFR4 reduced the sensitivity of HER2-positive breast cancer to trastuzumab plus pertuzumab or tucatinib. These results pinpoint a mechanism of anti-HER2 resistance and provide a strategy for overcoming resistance via FGFR4 inhibition in recalcitrant HER2-positive breast cancer. | ||||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | |||
| Responsed Drug | Tucatinib | ||||
| Pathway Response | Wnt signaling pathway | hsa04310 | |||
| Cell Process | Glutathione synthesis | ||||
In-vitro Model |
MDA-MB-453 | Breast adenocarcinoma | Homo sapiens | CVCL_0418 | |
| MDA-MB-361 | Breast adenocarcinoma | Homo sapiens | CVCL_0620 | ||
| SK-BR-3 | Breast adenocarcinoma | Homo sapiens | CVCL_0033 | ||
| BT-474 | Invasive breast carcinoma | Homo sapiens | CVCL_0179 | ||
| AU565 | Breast adenocarcinoma | Homo sapiens | CVCL_1074 | ||
| MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 | ||
| T-47D | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| ZR-75-1 | Invasive breast carcinoma | Homo sapiens | CVCL_0588 | ||
| MDA-MB-231 | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| BT-549 | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| MDA-MB-468 | Breast adenocarcinoma | Homo sapiens | CVCL_0419 | ||
| SUM159PT | Breast pleomorphic carcinoma | Homo sapiens | CVCL_5423 | ||
| MCF-10A | Normal | Homo sapiens | CVCL_0598 | ||
| HEK293T | Normal | Homo sapiens | CVCL_0063 | ||
| In-vivo Model | Luciferase-labeled rSKBR3 and MDA-MB-361 cells (1 × 107 cells) mixed with 1:1 Matrigel (Corning, 356237) were subcutaneously injected into the fat pads of mice. After a tumor was palpable, the mice were randomized into four groups (five mice per group), and they were treated with vehicle, trastuzumab (20 mg/kg, intraperitoneal administration), roblitinib (30 mg/kg, oral administration), or a combination of both drugs. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| DNA (cytosine-5)-methyltransferase 3B (DNMT3B) | 22 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Curcumin | Phase 3 | [3] | ||
| Synonyms |
458-37-7; Diferuloylmethane; Natural yellow 3; Turmeric yellow; Turmeric; Curcuma; Kacha haldi; Gelbwurz; Indian saffron; Curcumin I; Souchet; Halud; Halad; Haidr; Haldar; Merita earth; Yellow Ginger; Terra Merita; Yellow Root; Safran d'Inde; Yo-Kin; Golden seal; Curcuma oil; Orange Root; Oils, curcuma; CI Natural Yellow 3; Curcumine; Hydrastis; Indian turmeric; Yellow puccoon; Turmeric extract; Diferaloylmethane; Kurkumin [Czech]; (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione; Tumeric yellow; Turmeric oil
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27376512-Compound-MTC-424 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1940 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-427 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 295 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-422 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1430 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-423 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 363 nM | |||
| External Link | ||||
| NSC-622444 | Investigative | [3] | ||
| Synonyms |
NSC622444; CHEMBL116347; AC1Q3LXD; AC1L7GK5; SCHEMBL9755151; dichlorinated diacylmethane fragment; ZINC1616868; BDBM50048522; 5,3'-dicarboxy-4,4'-dihydrodiphenylmethane; 5,5''-methylenebis(3-chloro-2-hydroxybenzoic acid); 5,5'-Methylenebis(3-chloro-2-hydroxybenzoic acid); 3,3'-methanediylbis(5-chloro-6-hydroxybenzoic acid); 5-(3-carboxy-5-chloro-4-hydroxybenzyl)-3-chloro-2-hydroxybenzoic acid; 3',3-Dichloro-4',4-dimethoxy-5',5-bis(methoxycarbonyl)-1,1-diphenylmethane
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-138419 | Investigative | [3] | ||
| Synonyms |
NSC138419; n-[4-(methylamino)benzoyl]glutamic acid; AC1Q5SG8; AC1L5YW4; SCHEMBL5925511; CHEMBL591443; CTK1H0013; 2-[(4-methylaminobenzoyl)amino]pentanedioic acid; A816490; 2-[[4-(methylamino)benzoyl]amino]pentanedioic acid; 2-[[4-(methylamino)phenyl]carbonylamino]pentanedioic acid; 2-[[[4-(methylamino)phenyl]-oxomethyl]amino]pentanedioic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-319745 | Investigative | [3] | ||
| Synonyms |
61629-60-5; HB 093; BRN 2168571; 4-(2-((5-Chloro-2-methoxybenzoyl)amino)ethyl)hydrocinnamic acid; 3-[4-[2-[(5-CHLORO-2-METHOXY-BENZOYL)AMINO]ETHYL]PHENYL]PROPANOIC ACID; 3-(4-(2-(5-Chlor-2-methoxy-benzamido)-aethyl)phenyl)-propionsaeure [German]; 3-[4-[2-[(5-chloro-2-methoxybenzoyl)amino]ethyl]phenyl]propanoic acid; HYDROCINNAMIC ACID, 4-(2-((5-CHLORO-2-METHOXYBENZOYL)AMINO)ETHYL)-; AC1L2AFL; CHEMBL597112; SCHEMBL11481071; CTK5B3505; DTXSID00210642; AIEFQKOARQRACO-UHFFFAOYSA-N; ZINC1572211; HB-093; NSC319745
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-348926 | Investigative | [3] | ||
| Synonyms |
NSC348926; 2-phthalimidoadipic acid; AC1L7IP1; SCHEMBL9741723; CHEMBL599367; 2-(1,3-dioxoisoindol-2-yl)hexanedioic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-401077 | Investigative | [3] | ||
| Synonyms |
NSC401077; MLS000757170; DNA Methyltransferase Inhibitor; CHEMBL383475; 32675-71-1; 2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-3-(1H-indol-3-yl)-propionic acid; 2-(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)-3-(1H-indol-3-yl)propanoic acid; 2-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-3-(1H-indol-3-yl)propanoic acid; 2-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-3-(1H-indol-3-yl)propionic acid; SMR000413613; AC1Q71QA; Oprea1_475901; Oprea1_410805; MLS000777218; MLS006011919; SCHEMBL562060
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| S-tubercidinylhomocysteine | Investigative | [5] | ||
| Synonyms |
CHEMBL552309; 57344-98-6; AC1L3YAS; AC1Q5QMO; (S)-7-(5-S-(3-amino-3-carboxypropyl)-5-thio-beta-D-ribofuranosyl)-7H-pyrrolo(2,3-d)pyrimidin-4-amine; (2s)-2-amino-4-({[(2s,3s,4r,5r)-5-(4-amino-7h-pyrrolo[2,3-d]pyrimidin-7-yl)-3,4-dihydroxytetrahydrofuran-2-yl]methyl}sulfanyl)butanoic acid(non-preferred name); BDBM50294482; (2S)-2-amino-4-[[(2S,3S,4R,5R)-5-(4-aminopyrrolo[2,3-d]pyrimidin-7-yl)-3,4-dihydroxyoxolan-2-yl]methylsulfanyl]butanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 300 nM | |||
| External Link | ||||
| NSC-106084 | Investigative | [3] | ||
| Synonyms |
CHEMBL597113; NSC106084; AC1L6H8Q; CTK7J5419; ZINC1868549; BDBM50308983; {4-[5-bromo-2-(carboxymethoxy)benzoyl]phenoxy}acetic acid; 2-(4-bromo-2-(4-(carboxymethoxy)benzoyl)phenoxy)acetic acid; 2-[4-[5-bromo-2-(carboxymethyloxy)benzoyl]phenoxy]acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-154957 | Investigative | [3] | ||
| Synonyms |
NSC154957; AC1L6EF2; CHEMBL586418; 3-benzhydrylsulfanyl-2-formamidopropanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-54162 | Investigative | [3] | ||
| Synonyms |
NSC54162; AC1Q5WTA; AC1L6CM2; CHEMBL611994; 2,2'-{[(2-hydroxyphenyl)methanediyl]disulfanediyl}diacetic acid; ZINC1685025; Acetic acid, (salicylidenedithio)di-; 4265-51-4; Acetic acid, [(o-hydroxybenzylidene)dithio]di-; Acetic acid,2'-[[(2-hydroxyphenyl)methylene]bis(thio)]bis-; 2-[carboxymethylsulfanyl-(2-hydroxyphenyl)methyl]sulfanylacetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-57893 | Investigative | [3] | ||
| Synonyms |
MLS002667915; 7399-94-2; 4-[(1h-benzimidazol-2-ylmethyl)(formyl)amino]benzoic acid; NSC57893; AC1L6GFK; AC1Q5TWY; NCIOpen2_002368; CHEMBL599366; 4-[1H-benzimidazol-2-ylmethyl(formyl)amino]benzoic acid; CTK5D9099; DTXSID30288854; HMS3089M13; ZINC1688755; AKOS030547711
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-56071 | Investigative | [3] | ||
| Synonyms |
32230-52-7; NSC56071; AC1L6EJV; AC1Q7ES0; NCIOpen2_007380; CHEMBL596910; CTK4G8394; DTXSID80288485; ZINC1686711; 2,2'-[piperazine-1,4-diylbis(carbonothioylsulfanediyl)]diacetic acid; AKOS030574801; Acetic acid,2,2'-[1,4-piperazinediylbis(carbonothioylthio)]bis- (9CI); 2-[4-(carboxymethylsulfanylcarbothioyl)piperazine-1-carbothioyl]sulfanylacetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-137546 | Investigative | [3] | ||
| Synonyms |
NSC137546; CHEMBL591202; AC1L5Y49; AKOS008984447; 2-[(2,6-dichlorobenzoyl)amino]pentanedioic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-623548 | Investigative | [3] | ||
| Synonyms |
2581-36-4; NSC 408488; o-Cresotic acid, 5,5'-methylenedi-; 2,3-CRESOTIC ACID, 5,5'-METHYLENEDI-; UNII-S3D8KC88KC; 5,5'-Methylenedi-2,3-cresotic acid; NSC 623548; BRN 3433298; S3D8KC88KC; CHEMBL113835; 5,5'-Methylenedi-o-cresotic acid; NSC623548; NSC408488; 5,5'-Methylenebis(2-hydroxy-3-methylbenzoic acid); 2, 5,5'-methylenedi-; AC1L29YK; Oprea1_231968; 2-10-00-00398 (Beilstein Handbook Reference); SCHEMBL9755153; CTK4F6504; DTXSID90180466; o-Cresotic acid,5'-methylenedi-; MolPort-000-698-522; ZINC4028795; STL511095
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-345763 | Investigative | [3] | ||
| Synonyms |
7-(8-hydroxyquinolin-5-yl)-4,7-dioxoheptanoic acid; NSC345763; AC1L7HSU; CHEMBL597114
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-158324 | Investigative | [3] | ||
| Synonyms |
Acediasulfone; UNII-30YP2YHH8W; 30YP2YHH8W; CHEMBL48396; N-[4-[(4-AMINOPHENYL)SULPHONYL]PHENYL]GLYCINE; 2-[4-(4-aminophenyl)sulfonylanilino]acetic acid; Acediasulfonum; N-(4-((4-Aminophenyl)sulphonyl)phenyl)glycine; EINECS 201-243-7; AC1L25EF; ZINC862; SCHEMBL143660; CTK5E7379; DTXSID00229991; CHEBI:135300; BDBM50099670; AKOS027327086; DB08926; Glycine,N-[4-[(4-aminophenyl)sulfonyl]phenyl]-; {4-[(4-aminophenyl)sulfonyl]anilino}acetic acid; 2-(4-(4-aminophenylsulfonyl)phenylamino)acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (L-)-S-adenosyl-L-homocysteine | Investigative | [6] | ||
| Synonyms |
S-Adenosyl-L-homocysteine; S-adenosylhomocysteine; 979-92-0; AdoHcy; S-(5'-adenosyl)-L-homocysteine; adenosylhomocysteine; Formycinylhomocysteine; Adenosyl-L-homocysteine; S-(5'-deoxyadenosin-5'-yl)-L-homocysteine; 2-S-adenosyl-L-homocysteine; 5'-Deoxy-S-adenosyl-L-homocysteine; S-adenosyl-homocysteine; S-Adenosyl Homocysteine; L-S-Adenosylhomocysteine; L-Homocysteine, S-(5'-deoxyadenosin-5'-yl)-; adenosylhomo-cys; adenosyl-homo-cys; UNII-8K31Q2S66S; (S)-5'-(S)-(3-Amino-3-carboxypropyl)-5'-thioadenosine; BRN 5166233; SAH
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 200 nM | |||
| External Link | ||||
| NSC-622445 | Investigative | [3] | ||
| Synonyms |
5,5'-Methylenedisalicylic acid; 122-25-8; 5,5'-Methylenebis(2-hydroxybenzoic acid); Methylenebis(salicylic acid); 5,5-Methylenebis(salicylic acid); UNII-2KF4FVV76N; 5,5-Methylenedisalicylic acid; 5-(3-Carboxy-4-hydroxybenzyl)salicylic acid; 4,4'-Dihydroxy-3,3'-dicarboxydiphenylmethane; 3,3'-Dicarboxy-4,4'-dihydroxydiphenylmethane; NSC 14778; 2KF4FVV76N; 4,4'-Dihydroxydiphenylmethane-3,3'-dicarboxylic acid; 3,3'-Methylenebis(6-hydroxybenzoic acid); CHEMBL115145; Benzoic acid, 3,3'-methylenebis[6-hydroxy-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 17000 nM | |||
| External Link | ||||
| Glycogen synthase kinase-3 beta (GSK3Beta/GSK3B) | 88 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| AMO-02 | Phase 2/3 | [7] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Tideglusib | Phase 2 | [8] | ||
| Synonyms |
NP-031112; NP-12; NP031112; Tideglusib(NP-031112)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 78 nM | |||
| External Link | ||||
| 9-ING-41 | Phase 2 | [9] | ||
| Synonyms |
1034895-42-5; ND1SOF0DLU; UNII-ND1SOF0DLU; CHEMBL483465; 3-(5-fluoro-1-benzofuran-3-yl)-4-(5-methyl-[1,3]dioxolo[4,5-f]indol-7-yl)pyrrole-2,5-dione; 3-(5-fluorobenzofuran-3-yl)-4-(5-methyl-5H-[1,3]dioxolo[4,5-f]indol-7-yl)-1H-pyrrole-2,5-dione; elraglusib; SCHEMBL3152351; GTPL11412; EX-A4074; BDBM50267716; s9602; SB19735; compound 26 [PMID: 19338355]; HY-113914; CS-0063319; 1H-Pyrrole-2,5-dione, 3-(5-fluoro-3-benzofuranyl)-4-(5-methyl-5H-1,3-dioxolo(4,5-F)indol-7-yl)-; 3-(5-Fluoro-benzofuran-3-yl)-4-(5-methyl-5H-(1,3)dioxolo(4,5-F)indol-7-yl)-pyrrole-2,5-dione; 3-(5-Fluorobenzofuran-3-yl)-4-(5-methyl-5H-[1,3]dioxolo[4,5-f]indol-7-yl)pyrrole-2,5-dione; 4-(5-methyl-5H-[1,3]dioxolo[4,5-f]-indol-7-yl)-3-(5-fluoro-1-benzofuran-3-yl)-1 h-pyrrole-2,5-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| LY2090314 | Phase 2 | [10] | ||
| Synonyms |
603288-22-8; LY-2090314; UNII-822M3GYM67; Kinome_3681; LY 2090314; CHEMBL362558; 822M3GYM67; 3-(9-Fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetrahydro-[1,4]diazepino[6,7,1-hi]indol-7-yl)-4-(imidazo[1,2-a]pyridin-3-yl)-1H-pyrrole-2,5-dione; SCHEMBL633455; GTPL7958; DTXSID90209085; MolPort-035-944-332; EX-A2214; ZINC3817327; BCP07855; s7063; BDBM50150699; AKOS032950045; AKOS026750195; CS-1633; DB11913; SB16558; NCGC00378942-05; NCGC00378942-02; BC600682; QC-11735; HY-16294; KB-78238; FT-0698670; LY2090314, >
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 0.9 nM | |||
| External Link | ||||
| Lithium | Phase 2 | [11] | ||
| Synonyms |
7439-93-2; Li; litio; UNII-9FN79X2M3F; 9FN79X2M3F; CHEBI:30145; MFCD00134051; Litium; Lithium, metallic; Lithium, elemental; Lithium, 99+%, granular, dry; Lithium compounds; 3Li; HSDB 647; EINECS 231-102-5; UN1415; monolithium; Lithium standard solution, for AAS, 1 mg-ml Li in 2% HCl; Hydrure de lithium [French]; Lithium ribbon; Lithium rod; HSDB 549; Lithium granules; EINECS 231-484-3; UN1414; UN2805; Normothymin-E (TN); Epitope ID:114079; EC 231-102-5; Lithium, 99%, low sodium; Lithium, 99%, high sodium; CHEMBL2146126; DTXSID5036761; HSDB 6900; 7321AH; AKOS015833388; AKOS015902481; HSD6900000; Lithium wire, 3.2mm (0.125in) dia; Lithium [UN1415] [Dangerous when wet]; Lithium granules, 1-6mm (0.04-0.2in); Lithium, granular, 99% trace metals basis; FT-0627905; C15473; D08133; EC 231-484-3; Lithium hydride [UN1414] [Dangerous when wet]; Lithium, shot, 99%, 4-16 mesh, in mineral oil; Lithium, wire, diam. 3.2 mm, in mineral oil, >=98%; Lithium, ~25 wt % dispersion in mineral oil, high sodium; Lithium, AAS standard solution, Specpure?, Li 1000?g/ml; Lithium, ingot, diam. 5.7 cm, 99.9% trace metals basis; Lithium, rod, diam. 12.7 mm, 99.9% trace metals basis; Lithium foil, 0.75mm (0.03in) thick x 19mm (0.75in) wide; Lithium hydride, fused solid [UN2805] [Dangerous when wet]; Lithium ingot, 5.7cm (2.2in) dia x 8.6cm (3.4in) long; Lithium, Oil based standard solution, Specpure, Li 5000g/g; Lithium, plasma standard solution, Specpure?, Li 10,000?g/ml; Lithium, plasma standard solution, Specpure?, Li 1000?g/ml; Lithium, rod, 12.7 mm diameter, length 165 mm, purity 99%; Lithium, rod, 12.7 mm diameter, length 200 mm, purity 99%; Lithium, foil, 25x100mm, thickness 0.6mm, as rolled, 99.9%; Lithium, Oil based standard solution, Specpure(R), Li 1000?g/g; Lithium, foil, thickness 0.6 mm, size 25 x 300 mm, purity 99.9%; Lithium, Ion chromatography standard solution, Specpure, Li+ 1000?g/ml; Lithium, wire (in mineral oil), diam. 3.2 mm, 99.9% trace metals basis; Lithium, foil, not light tested, 38x200mm, thickness 0.20mm, as rolled, 99.9%; Lithium, foil, not light tested, 38x500mm, thickness 0.20mm, as rolled, 99.9%; Lithium, foil, not light tested, 45x200mm, thickness 0.12mm, as rolled, 99.9%; Lithium, granular, 4-10 mesh particle size, high sodium, 99% (metals basis); Lithium, ribbon, thickness x W 0.38 mm x 23 mm, 99.9% trace metals basis; Lithium, ribbon, thickness x W 0.75 mm x 19 mm, 99.9% trace metals basis; Lithium, ribbon, thickness x W 0.75 mm x 45 mm, 99.9% trace metals basis; Lithium, ribbon, thickness x W 1.5 mm x 100 mm, 99.9% trace metals basis
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Neu-120 | Phase 1/2 | [12] | ||
| Synonyms |
Neu-108; NMDA receptor modulators (Parkinson's Disease), Neurim
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| TDZD-8 | Patented | [13] | ||
| Synonyms |
327036-89-5; 4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione; GSK-3beta Inhibitor I; TDZD 8; 1,2,4-Thiadiazolidine-3,5-dione, 2-methyl-4-(phenylmethyl)-; MFCD04973552; NP 01139; AK-48153; 4-Benzyl-2-methyl-[1,2,4]thiadiazolidine-3,5-dione; 1,2,4-Thiadiazolidine-3,5-dione,2-methyl-4-(phenylmethyl)-; GSK-3 Inhibitor I; SCHEMBL139834; GTPL5977; CHEMBL284861; BDBM7781; CTK4G9152; ZINC27361; AOB6176; EX-A109; DTXSID30399590; JDSJDASOXWCHPN-UHFFFAOYSA-N; MolPort-003-844-896; HMS3229G12; A Inhibitor I, TDZD-8
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 690 nM | |||
| External Link | ||||
| AR-A014418 | Patented | [14] | ||
| Synonyms |
487021-52-3; GSK-3beta Inhibitor VIII; 1-(4-methoxybenzyl)-3-(5-nitrothiazol-2-yl)urea; 1-[(4-methoxyphenyl)methyl]-3-(5-nitro-1,3-thiazol-2-yl)urea; A Inhibitor VIII; N-(4-METHOXYBENZYL)-N'-(5-NITRO-1,3-THIAZOL-2-YL)UREA; UNII-87KSH90Q6D; AR-AO 14418; SN 4521; AR-A 014418; CHEMBL259850; 87KSH90Q6D; N-[(4-Methoxyphenyl)methyl]-N'-(5-nitro-2-thiazolyl)urea; AK175829; C12H12N4O4S; N-(4-Methoxybenzyl)-N& -(5-nitro-1,3-thiazol-2-yl)urea; AR 014418; GSK 3be
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.07 nM | |||
| External Link | ||||
| PMID26161698-Compound-18 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = high nM | |||
| External Link | ||||
| CHIR-99021 | Patented | [16] | ||
| Synonyms |
CHIR99021; CHIR 99021; CT-99021; CT99021
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 30 nM | |||
| External Link | ||||
| KENPAULLONE | Patented | [17] | ||
| Synonyms |
142273-20-9; 9-Bromopaullone; NSC 664704; 9-Bromo-7,12-dihydroindolo[3,2-d][1]benzazepin-6(5H)-one; NSC-664704; NSC664704; MLS002702152; CHEMBL296586; 9-Bromo-7,12-dihydroindolo(3,2-d)(1)benzazepin-6(5H)-one; Indolo[3,2-d][1]benzazepin-6(5H)-one,9-bromo-7,12-dihydro-; QQUXFYAWXPMDOE-UHFFFAOYSA-N; 9-Bromo-7,12-dihydro-indolo[3,2-d][1]benzazepin-6(5H)-one; 9-bromo-7,12-dihydro-5H-indolo[3,2-d][1]benzazepin-6-one; 9-Bromo-7,12-dihydro-indolo-[3,2-d]-[1]benzazepin-6(5H)-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 23 nM | |||
| External Link | ||||
| AZD-1080 | Discontinued in Phase 1 | [18] | ||
| MOA | Modulator | |||
| Activity | IC50 = 31 nM | |||
| External Link | ||||
| SAN-61 | Terminated | [19] | ||
| Synonyms |
SAN-AL-61; Oral beta amyloid inhibitor (Alzheimer's disease), Sanomune
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RO-320432 | Terminated | [20] | ||
| Synonyms |
ro 32-0432; Ro-32-0432; CHEMBL26501; (S)-3-(8-(Dimethylaminomethyl)-6,7,8,9-tetrahydropyrido(1,2-a)indol-10-yl)-4-(1-methyl-3-indolyl)-1H-pyrrole-2,5-dione hydrochloride; (S)-3-(8-((Dimethylamino)methyl)-6,7,8,9-tetrahydropyrido(1,2-a)indol-10-yl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrol-2,5-dione; (S)-3-(8-((Dimethylamino)methyl)-6,7,8,9-tetrahydropyrido(1,2-a)indol-10-yl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione; 151342-35-7; 1H-Pyrrole-2,5-dione, 3-(8-((dimethylamino)methyl)-6,7,8,9-tetrahydropyrido(1,2-a)in
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| 8-O-(4-chlorobenzenesulfonyl)manzamine F | Investigative | [21] | ||
| Synonyms |
CHEMBL400717
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7200 nM | |||
| External Link | ||||
| N,8-diphenyl-9H-purin-6-amine | Investigative | [22] | ||
| Synonyms |
CHEMBL1210175; N,8-Diphenyl-9H-purine-6-amine; BDBM50322830; SR-01000661492
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 16 nM | |||
| External Link | ||||
| N-(8-(3-cyanophenyl)-9H-purin-6-yl)pentanamide | Investigative | [22] | ||
| Synonyms |
CHEMBL1210476
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1220 nM | |||
| External Link | ||||
| N-(6-(3-hydroxyphenyl)-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
CHEMBL1086639; SCHEMBL6485118; UKQRPDHIQLHFNS-UHFFFAOYSA-N; BDBM50313706
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 94 nM | |||
| External Link | ||||
| GSK-3beta inhibitor II | Investigative | [24] | ||
| Synonyms |
GSK-3b inhibitor II
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 390 nM | |||
| External Link | ||||
| 3-phenyl-4-(phenylamino)-1H-pyrrole-2,5-dione | Investigative | [25] | ||
| Synonyms |
SKF-62604; 4-arylmaleimide deriv. 6a; phenyl anilino-maleimide; AC1O6ZNQ; BDBM8218; SCHEMBL5682682; CHEMBL346551; HMS3303J01; HMS3305A03; 2-(Phenylamino)-3-phenylmaleimide; 3-anilino-4-phenylpyrrole-2,5-dione; NCGC00241898-01; AB01092118-01
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 520 nM | |||
| External Link | ||||
| 8-O-(4-bromobenzenesulfonyl)manzamine F | Investigative | [21] | ||
| Synonyms |
CHEMBL414128
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 23000 nM | |||
| External Link | ||||
| I-5 | Investigative | [26] | ||
| Synonyms |
SB-409513; 2-CHLORO-5-[4-(3-CHLORO-PHENYL)-2,5-DIOXO-2,5-DIHYDRO-1H-PYRROL-3-YLAMINO]-BENZOIC ACID; 2-chloro-5-{[4-(3-chlorophenyl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]amino}benzoic acid; AC1L9LHU; 4-arylmaleimide deriv. 6-z; CHEMBL156987; BDBM8269; SCHEMBL10059345; HMS3303B04; HMS3305M22; DB01793; NCGC00241937-01; AB01092116-01; 2-(3-Carboxy-4-chlorophenylamino)-3-(3-chlorophenyl)maleimide; 2-chloro-5-[[4-(3-chlorophenyl)-2,5-dioxopyrrol-3-yl]amino]benzoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 76 nM | |||
| External Link | ||||
| GSK-3beta inhibitor XI | Investigative | [27] | ||
| Synonyms |
GSK-3b inhibitor XI
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| Manzamine E | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(6-(4-fluorophenyl)-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
CHEMBL1084681; SCHEMBL4488738; ORRUGYIZWSJRJU-UHFFFAOYSA-N; BDBM50313680
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5008 nM | |||
| External Link | ||||
| N-(6-(2-chlorophenyl)-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
CHEMBL1086175; N-[6-(2-chlorophenyl)-1H-indazol-3-yl]butanamide; SCHEMBL6485112; WPJGFQOEGTUKTE-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1353 nM | |||
| External Link | ||||
| N-(6-bromo-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
CHEMBL1086780; N-[6-bromo-1H-indazol-3-yl]butanamide; 599191-53-4; butanamide,n-(6-bromo-1h-indazol-3-yl)-; SCHEMBL6490286; QQVNXRROZHWJNS-UHFFFAOYSA-N; ZINC38816494; BDBM50313708
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 198 nM | |||
| External Link | ||||
| Manzamine Y | Investigative | [28] | ||
| Synonyms |
CHEBI:66668; (4aR,7S,7aR,13Z,14aR,15aR,18Z)-5-(6-hydroxy-9H-beta-carbolin-1-yl)-4,4a,9,10,11,12,14a,15-octahydro-3H-7,2-oct[3]enoazocino[1',2':1,5]pyrrolo[2,3-i]isoquinolin-7(1H,7aH)-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| IM-12 | Investigative | [29] | ||
| Synonyms |
1129669-05-1; CHEMBL1254896; 3-(4-Fluorophenylethylamino)-1-methyl-4-(2-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione; 3-(4-fluorophenethylamino)-1-methyl-4-(2-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione; 3-[2-(4-fluorophenyl)ethylamino]-1-methyl-4-(2-methyl-1H-indol-3-yl)pyrrole-2,5-dione; IM 12; GTPL8017; SCHEMBL17378682; DTXSID20649091; AOB4090; MolPort-035-789-694; CHEBI:125616; HMS3653F15; BCP10769; EX-A2071; ZINC59086693; s7566; BDBM50326901; 2472AH; AKOS026750397; SB19269; CS-3399; CCG-208085; NCGC00386352-05; BC600587
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 53 nM | |||
| External Link | ||||
| N-(6-phenethyl-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
CHEMBL1084684; SCHEMBL6489338
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8525 nM | |||
| External Link | ||||
| N-(6-benzyl-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
CHEMBL1077260; SCHEMBL6490520
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4140 nM | |||
| External Link | ||||
| (2'Z,3'E)-7-Azaindirubin-3'-oxime | Investigative | [30] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(6-phenyl-1H-indazol-3-yl)butyramide | Investigative | [31] | ||
| Synonyms |
CHEMBL1086174; SCHEMBL4493121
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 390 nM | |||
| External Link | ||||
| Neo-kauluamine | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| (E)-N-(6-(prop-1-enyl)-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
CHEMBL1086176
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2459 nM | |||
| External Link | ||||
| 8-OH-MANZAMINE A | Investigative | [28] | ||
| Synonyms |
8-Hydroxymanzamine A; 154466-37-2; (4aR,7S,7aR,13Z,14aR,15aR,18Z)-5-(8-hydroxy-9H-beta-carbolin-1-yl)-4,4a,9,10,11,12,14a,15-octahydro-3H-7,2-oct[3]enoazocino[1',2':1,5]pyrrolo[2,3-i]isoquinolin-7(1H,7aH)-ol; (+)8-Hydroxymanzamine A; CHEBI:66669
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-deoxymanzamine X | Investigative | [21] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(6-(4-aminophenyl)-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
CHEMBL1086638; N-[6-(4-aminophenyl)-1H-indazol-3-yl]butanamide; SCHEMBL6478145; DCCRABAZYMVLJU-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 76 nM | |||
| External Link | ||||
| N-(6-(trifluoromethyl)-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
N-[6-(trifluoromethyl)-1H-indazol-3-yl]butanamide; CHEMBL1086640; 599191-49-8; SCHEMBL1462440; RVYSDAGRNRYYML-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 195 nM | |||
| External Link | ||||
| PAULLONE | Investigative | [20] | ||
| Synonyms |
142273-18-5; 7,12-Dihydroindolo[3,2-d][1]benzazepin-6(5H)-one; CHEMBL430574; NSC641166; NSC 641166; 7,12-dihydro-5H-indolo[3,2-d][1]benzazepin-6-one; 8,18-diazatetracyclo[9.7.0.0; {12,17}]octadeca-1(11),2,4,6,12(17),13,15-heptaen-9-one; AC1Q6O0K; AC1L7YZ4; BDBM7287; SCHEMBL3178594; CTK4C3026; DTXSID30327277; CHEBI:138487; ZINC1626613; AKOS024113922; NSC-641166; NCI60_013826; RT-014947; FT-0673529
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| 12,13-DEHYDRO-8-O-ACETYLMANZAMINE A | Investigative | [21] | ||
| Synonyms |
CHEMBL252520
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4800 nM | |||
| External Link | ||||
| N-(6-(pyridin-4-yl)-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
CHEMBL1086782; SCHEMBL1460286; BBNQSWZLWJBJIN-UHFFFAOYSA-N; BDBM50313652
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 661 nM | |||
| External Link | ||||
| 4-(5-bromo-1H-indol-3-yl)pyrimidin-2-amine | Investigative | [14] | ||
| Synonyms |
Meridianin C; 213473-00-8; 2-Pyrimidinamine, 4-(5-bromo-1H-indol-3-yl)-; CHEMBL44541; SCHEMBL1612228; CTK0J7680; BDBM10840; DTXSID50434275; PKQJCYXKRNGUKQ-UHFFFAOYSA-N; AKOS027469387; AS-49872
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2000 nM | |||
| External Link | ||||
| 9-N-METHYL-8-METHOXY-MANZAMINE A | Investigative | [21] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(6-(furan-3-yl)-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
CHEMBL1086781; SCHEMBL1461525; ZGQZPFJWHPTRAH-UHFFFAOYSA-N; BDBM50313709
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 260 nM | |||
| External Link | ||||
| N-(6-chloro-5-p-tolyl-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
CHEMBL1097694; SCHEMBL1461361; REESNWQQNIKZJK-UHFFFAOYSA-N; BDBM50313666
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 14000 nM | |||
| External Link | ||||
| 12,13-DEHYDROMANZAMINE A | Investigative | [21] | ||
| Synonyms |
CHEMBL268202
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5400 nM | |||
| External Link | ||||
| CT-98024 | Investigative | [20] | ||
| Synonyms |
556813-39-9; N2-(2-((4-(2,4-Dichlorophenyl)-5-(1H-imidazol-2-yl)pyrimidin-2-yl)amino)ethyl)-5-nitropyridine-2,6-diamine; CHEMBL1080901; CHIR-98024; N6-[2-[[4-(2,4-Dichlorophenyl)-5-(1H-imidazol-2-yl)-2-pyrimidinyl]amino]ethyl]-3-nitro-2,6-pyridinediamine; NDFXSHIIGXVOKT-UHFFFAOYSA-N; SCHEMBL4394521; CTK8E8250; DTXSID90433308; MolPort-006-393-166; BCP13710; BDBM50313013; ABP000489; ZINC44136098; AKOS016011284; KB-76042; CHIR98014(CT98014)/; AK120785; RT-011992; AX8246201; AJ-109287; FT-0664506; Z-3284
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.56 nM | |||
| External Link | ||||
| N-(6-(pyridin-3-yl)-1H-indazol-3-yl)butyramide | Investigative | [31] | ||
| Synonyms |
CHEMBL1085918; SCHEMBL6489919; CZTAWAQITKVBNQ-UHFFFAOYSA-N; BDBM50313686
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5900 nM | |||
| External Link | ||||
| N-(6-chloro-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
CHEMBL1082235; N-(6-chloro-1H-indazol-3-yl)butanamide; SCHEMBL4497072; VHQZZDZWVQELLJ-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 354 nM | |||
| External Link | ||||
| 9-N-ETHYL-8-ETHOXY-MANZAMINE A | Investigative | [21] | ||
| Synonyms |
CHEMBL403562
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10400 nM | |||
| External Link | ||||
| N-(6-(4-hydroxyphenyl)-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 44 nM | |||
| External Link | ||||
| N-(6-(thiophen-3-yl)-1H-indazol-3-yl)butyramide | Investigative | [23] | ||
| Synonyms |
CHEMBL1083208; SCHEMBL1462380; KEXXQGBZRBKVRC-UHFFFAOYSA-N; BDBM50313653
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 935 nM | |||
| External Link | ||||
| CP-70949 | Investigative | [32] | ||
| Synonyms |
PFI-367; PFI-856; GSK-3-beta inhibitor, Pfizer; Glycogen synthase kinase-3-beta inhibitor, Pfizer
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(6-(phenylamino)-9H-purin-8-yl)benzonitrile | Investigative | [22] | ||
| Synonyms |
CHEMBL1210236; BDBM50322829
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 26 nM | |||
| External Link | ||||
| DM-204 | Investigative | [32] | ||
| Synonyms |
Anti-GSK-3beta mAbs, DiaMedica; Anti-GSK-3beta mAbs, Sanomune; Anti-GSK-3beta monoclonal antibodies, DiaMedica; Anti-GSK-3beta monoclonal antibodies, Sanomune
Click to Show/Hide
|
|||
| External Link | ||||
| CHIR-98023 | Investigative | [14] | ||
| Synonyms |
CT-98014
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 0.09 nM | |||
| External Link | ||||
| 8-O-(4-toluenesulfonyl)manzamine A | Investigative | [21] | ||
| Synonyms |
CHEMBL403561
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(6-chloro-5-phenyl-1H-indazol-3-yl)butyramide | Investigative | [31] | ||
| Synonyms |
CHEMBL1095040; N-(6-chloro-5-phenyl-1H-indazol-3-yl)butanamide; 3lfs; SCHEMBL1461347; WGVVIVGNBSSANI-UHFFFAOYSA-N; BDBM50313661; N-(5-phenyl-6-chloro-1H-indazol-3-yl)butanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 80 nM | |||
| External Link | ||||
| CHIR-98014 | Investigative | [16] | ||
| Synonyms |
CHIR98014; CHIR 98014
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.6 nM | |||
| External Link | ||||
| alsterpaullone 2-cyanoethyl | Investigative | [33] | ||
| Synonyms |
alsterpaullone derivative7
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.8 nM | |||
| External Link | ||||
| indirubin deriv. 8a | Investigative | [34] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| LEUCETTAMINE B | Investigative | [35] | ||
| Synonyms |
CHEMBL485053; SCHEMBL13219029
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SB-415286 | Investigative | [14] | ||
| Synonyms |
SB 415286; 264218-23-7; SB415286; 3-(3-chloro-4-hydroxyphenylamino)-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione; 3-(3-chloro-4-hydroxyphenylamino)-4-(4-nitrophenyl)-1H-pyrrole-2,5-dione; CHEMBL322970; 3-(3-chloro-4-hydroxyanilino)-4-(2-nitrophenyl)pyrrole-2,5-dione; 3-[(3-chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione; 1H-Pyrrol-2,5-dione, 3-((3-chloro-4-hydroxyphenyl)amino)-4-(2-nitrophenyl)-; SMR000568415; SR-01000075855
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 24 nM | |||
| External Link | ||||
| MANZAMINE A | Investigative | [36] | ||
| Synonyms |
104196-68-1; CHEMBL611781; SCHEMBL11915472
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8400 nM | |||
| External Link | ||||
| PYRAZOLOPYRIDAZINE 1 | Investigative | [37] | ||
| Synonyms |
551920-54-8; GW810576X; n-(3-methoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-yl-2-pyrimidinamine; pyrazolo[1,5-b]pyridazine deriv. 19; AC1O6ZIQ; CHEMBL187081; BDBM8128; SCHEMBL4489357; CTK1F7320; DTXSID60424889; HMS3305F24; HMS3303K24; ZINC13582569; NCGC00242229-01; DA-42106; FT-0707969; AB01092291-01; 2-Pyrimidinamine, N-(3-methoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-yl-; N-(3-methoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-ylpyrimidin-2-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 10 nM | |||
| External Link | ||||
| RGB-286147 | Investigative | [33] | ||
| Synonyms |
pyrazolopyrimidone analog, RGB-286147
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 754 nM | |||
| External Link | ||||
| PYRAZOLOPYRIDAZINE 2 | Investigative | [37] | ||
| Synonyms |
pyrazolo[1,5-b]pyridazine deriv. 25; AC1O6ZJ2; BDBM8134; CHEMBL186054; N-(3,4-dimethoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-ylpyrimidin-2-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 10 nM | |||
| External Link | ||||
| K00244 | Investigative | [38] | ||
| Synonyms |
GSK-3 Inhibitor XIII; N-(5-methyl-1H-pyrazol-3-yl)-2-phenylquinazolin-4-amine; GSK3-XIII; 404828-08-6; CHEMBL359482; (5-Methyl-1H-pyrazol-3-yl)-(2-phenylquinazolin-4-yl)amine; CHEBI:78544; AC1O4WD1; SCHEMBL462877; GTPL5976; CTK4I3154; HMS3229I06; BDBM228657; BCP12434; BDBM50162083; ZINC16052235; IN1311; AKOS025394676; DB08454; CCG-101293; NCGC00387776-01; ACM404828086; RT-013126; N-(3-methyl-1H-pyrazol-5-yl)-2-phenylquinazolin-4-amine; (5-Methyl-1H-pyrazol-3-yl)-(2-phenyl-quinazolin-4-yl)-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 22 nM | |||
| External Link | ||||
| Quinoxaline1 | Investigative | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1000 nM | |||
| External Link | ||||
| TWS-119 | Investigative | [39] | ||
| Synonyms |
TWS119; 601514-19-6; 3-[[6-(3-Aminophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]oxy]phenol; TWS 119; GSK inhibitor XII; GSK-3beta Inhibitor XII, TWS119; Neurogenesis Inducer, TWS119; CHEMBL405759; 3-(6-(3-aminophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yloxy)phenol; 3-((6-(3-AMINOPHENYL)-7H-PYRROLO[2,3-D]PYRIMIDIN-4-YL)OXY)PHENOL; 3-{[6-(3-aminophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]oxy}phenol; Phenol, 3-[[6-(3-aminophenyl)-1H-pyrrolo[2,3-d]pyrimidin-4-yl]oxy]-; K00245; MLS006011018; GTPL5980; SCHEMBL5559045; GSK-3BETA INHIB
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 30 nM | |||
| External Link | ||||
| AS-601245 | Investigative | [40] | ||
| Synonyms |
JNK Inhibitor V; SAPK Inhibitor V; 1,3-Benzothiazol-2-yl-(2-((2-(3-pyridinyl)ethyl)amino)-4-pyrimidinyl)acetonitrile; GTPL5997; SCHEMBL12242792; MolPort-044-724-552; HMS3229I20; CCG-206858; RT-013405; J-019673
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| AZAKENPAULLONE | Investigative | [17] | ||
| Synonyms |
1-Azakenpaullone; 676596-65-9; 9-bromo-7,12-dihydropyrido[3',2':2,3]azepino[4,5-b]indol-6(5H)-one; C15H10BrN3O; Kinome_3492; 1-AKP; SCHEMBL378920; GTPL8018; CHEMBL336961; BDBM7497; DTXSID8042686; NOCAS_42686; CTK8F0375; MolPort-003-844-675; CHEBI:131490; HMS3653A17; HMS3229B07; BCP21061; ZINC13588927; s7193; 2138AH; 1-Azakenpaullone, > AKOS030240424; SB19270; TRA0006688; NCGC00386322-01; RT-006179; FT-0662368; SW220021-1; KS-00001866
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 18 nM | |||
| External Link | ||||
| Thieno analogue of kenpaullone | Investigative | [17] | ||
| Synonyms |
Paullone Analogue 71; CHEMBL323657; SCHEMBL5688610; BDBM7336; NSC 703058; 8-Bromo-6,11-dihydro-thieno[3 ,2 :2,3]azepino[4,5-b]indol-5(4H)-one; 9-Bromo-5,4-(epithioetheno)-1,2,3,6-tetrahydroazepino[4,5-b]indole-2-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 119.95 nM | |||
| External Link | ||||
| L-779450 | Investigative | [41] | ||
| Synonyms |
303727-31-3; L-779,450; 2-chloro-5-(2-phenyl-5-(pyridin-4-yl)-1H-imidazol-4-yl)phenol; L779450; CHEMBL373011; 2-chloro-5-(2-phenyl-5-pyridin-4-yl-1H-imidazol-4-yl)phenol; 2-(Phenyl)-4-(3-hydroxy-4-chlorophenyl)-5-(4-pyridyl)-1H-imidazole; 2-Chloro-5-[2-phenyl-5-(4-pyridinyl)-1H-imidazol-4-yl]phenol; C20H14ClN3O; L 779450; 2-chloro-5-[2-phenyl-5-(pyridin-4-yl)-1H-imidazol-4-yl]phenol; 2-chloro-5-[2-phenyl-4-(pyridin-4-yl)-1H-imidazol-5-yl]phenol; 2-chloro-5-(2-phenyl-4-(pyridin-4-yl)-1H-imidazol-5-yl)phenol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NU-6102 | Investigative | [42] | ||
| Synonyms |
nu6102; 444722-95-6; NU 6102; O6-CYCLOHEXYLMETHOXY-2-(4'-SULPHAMOYLANILINO) PURINE; Cdk1/2 Inhibitor II, NU6102; 6-Cyclohexylmethoxy-2-(4& -sulfamoylanilino)purine; 4-{[6-(cyclohexylmethoxy)-9H-purin-2-yl]amino}benzenesulfonamide; 4SP; 1h1s; 4-[[6-(cyclohexylmethoxy)-7H-purin-2-yl]amino]benzenesulfonamide; 4-{[6-(cyclohexylmethoxy)-7h-purin-2-yl]amino}benzenesulfonamide; 4-[[6-(cyclohexylmethoxy)-9h-purin-2-yl]amino]benzenesulfonamide; 4eor; 4eok; 2iw9; 2c6o; 2iw8; AC1L1IGA; SCHEMBL2170816; CHEMBL319467
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 40 nM | |||
| External Link | ||||
| PF-228 | Investigative | [43] | ||
| Synonyms |
869288-64-2; PF-573228; PF 573228; PF573228; CHEMBL514554; 3,4-Dihydro-6-[[4-[[[3-(methylsulfonyl)phenyl]methyl]amino]-5-(trifluoromethyl)-2-pyrimidinyl]amino]-2(1H)-quinolinone; 6-((4-((3-(Methylsulfonyl)benzyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-3,4-dihydroquinolin-2(1H)-one; 6-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluoromethyl)pyrimidin-2-ylamino)-3,4-dihydroquinolin-2(1H)-one; 6-[4-(3-Methanesulfonyl-benzylamino)-5-trifluoromethyl-pyrimidin-2-ylamino]-3,4-dihydro-1H-quinolin-2-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 > 1000 nM | |||
| External Link | ||||
| BX-912 | Investigative | [44] | ||
| Synonyms |
BX 912; BX912
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BX-795 | Investigative | [44] | ||
| Synonyms |
BX795; BX 795
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID19115845C89S | Investigative | [45] | ||
| Synonyms |
3du8; GTPL8114; BDBM27380; DB07149
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 146 nM | |||
| External Link | ||||
| STAUROSPORINONE | Investigative | [46] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Ro31-8220 | Investigative | [46] | ||
| Synonyms |
Bisindolylmaleimide IX; ro 31-8220; 125314-64-9; Ro 31 8220; Ro 318220; UNII-W9A0B5E78O; Ro-318220; Ro-31-8220; CHEMBL6291; W9A0B5E78O; CHEBI:38912; 3-{3-[4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-1H-indol-1-yl}propyl carbamimidothioate; 3-{3-[4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-1H-indol-1-yl}propyl imidothiocarbamate; CHEMBL1591531; Carbamimidothioic acid, 3-(3-(2,5-dihydro-4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-1H-pyrrol-3-yl)-1H-indol-1-yl)propyl; bisindolymaleimide IX
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.8 nM | |||
| External Link | ||||
| AMP-PNP | Investigative | [47] | ||
| Synonyms |
Phosphoaminophosphonic acid-adenylate ester; gamma-Imino-ATP; ADENYLYL IMIDODIPHOSPHATE; AMPPNP; Adenyl imidodiphosphate; 25612-73-1; adenyl-5'-yl imidodiphosphate; CHEBI:47785; App(NH)p; O(5')-(1,2-dihydroxy-2-phosphonoaminodiphosphoryl)adenosine; 5'-O-(hydroxy{[hydroxy(phosphonoamino)phosphoryl]oxy}phosphoryl)adenosine; [[[[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]amino]phosphonic acid; p(NH)Ppf; beta,gamma-Imido-ATP; beta,gamma-Imidoadenosine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ELLAGIC ACID | Investigative | [48] | ||
| Synonyms |
476-66-4; Benzoaric acid; Lagistase; Eleagic acid; Alizarine Yellow; Elagostasine; 2,3,7,8-Tetrahydroxychromeno[5,4,3-cde]chromene-5,10-dione; Ellagic acid dihydrate; Llagic acid; Acide ellagique; Acido elagico; Acidum ellagicum; C.I. 55005; Gallogen (VAN); Gallogen (astringent); C.I. 75270; Ellagate; Ellagic acid [INN:DCF]; UNII-19YRN3ZS9P; Acido elagico [INN-Spanish]; CCRIS 774; Gallogen, astringent; Acide ellagique [INN-French]; Acidum ellagicum [INN-Latin]; MLS000069632; C14H6O8; EINECS 207-508-3; NSC407286; NSC 40728
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7500 nM | |||
| External Link | ||||
| CI-1040 | Investigative | [46] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 4,5,6,7-tetrabromo-1H-benzo[d][1,2,3]triazole | Investigative | [49] | ||
| Synonyms |
4,5,6,7-tetrabromobenzotriazole
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-[(3,5-diamino-1H-pyrazol-4-yl)diazenyl]phenol | Investigative | [50] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Bisindolylmaleimide-I | Investigative | [46] | ||
| Synonyms |
Bisindolylmaleimide i; 133052-90-1; GF 109203X; GF109203X; Go 6850; GF-109203X; RBT205 INHIBITOR; Go-6850; UNII-L79H6N0V6C; Bisindolylmaleimide I (GF 109203X); CHEMBL7463; 3-{1-[3-(DIMETHYLAMINO)PROPYL]-1H-INDOL-3-YL}-4-(1H-INDOL-3-YL)-1H-PYRROLE-2,5-DIONE; 3-(1-(3-(Dimethylamino)propyl)-1H-indol-3-yl)-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione; L79H6N0V6C; QMGUOJYZJKLOLH-UHFFFAOYSA-N; 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl)maleimide; GF-109203; Go6850
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 190.55 nM | |||
| External Link | ||||
| 2C60: Breast cancer | 2 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Entrectinib | Approved | [51] | ||
| Synonyms |
1108743-60-7; RXDX-101; UNII-L5ORF0AN1I; Entrectinib (RXDX-101); L5ORF0AN1I; Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-; Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-; Entrectinib [USAN:INN]; YMX; Kinome_2659; Entrectinib(rxdx-101); Entrectinib (USAN/INN); SCHEMBL3512601; GTPL8290; CHEMBL1983268; KS-00000TSK
Click to Show/Hide
|
|||
| External Link | ||||
| Everolimus | Approved | [52] | ||
| External Link | ||||
References