m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00708)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
NLRP1
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Methyltransferase-like 3 (METTL3) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL3 | ||
| Cell Line | HUVEC cell line | Homo sapiens |
|
Treatment: shMETTL3 HUVEC cells
Control: shScramble HUVEC cells
|
GSE157544 | |
| Regulation |
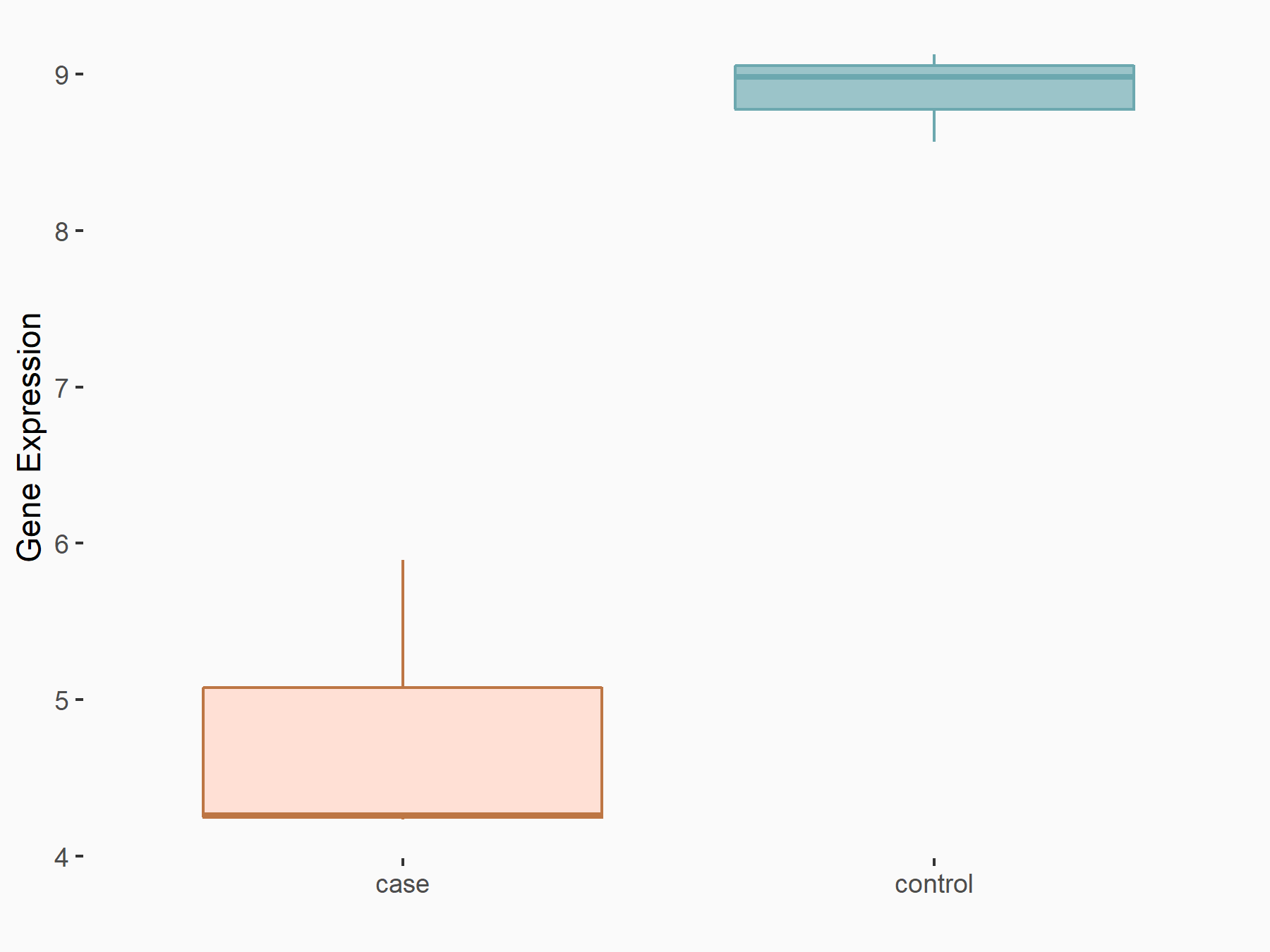  |
logFC: -7.83E-01 p-value: 2.96E-03 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | In the in vivo atherosclerosis model,partial ligation of the carotid artery led to plaque formation and up-regulation of METTL3 and NACHT/LRR/PYD domains-containing protein 1 (NLRP1), with down-regulation of KLF4; knockdown of METTL3 via repetitive shRNA administration prevented the atherogenic process, NLRP3 up-regulation, and KLF4 down-regulation. Collectively, it has demonstrated that METTL3 serves a central role in the atherogenesis induced by oscillatory stress and disturbed blood flow. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Atherosclerosis | ICD-11: BD40.Z | ||
| In-vitro Model | MAEC | Normal | Mus musculus | CVCL_U411 |
| HUVEC-C | Normal | Homo sapiens | CVCL_2959 | |
Atherosclerosis [ICD-11: BD40]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | In the in vivo atherosclerosis model,partial ligation of the carotid artery led to plaque formation and up-regulation of METTL3 and NACHT/LRR/PYD domains-containing protein 1 (NLRP1), with down-regulation of KLF4; knockdown of METTL3 via repetitive shRNA administration prevented the atherogenic process, NLRP3 up-regulation, and KLF4 down-regulation. Collectively, it has demonstrated that METTL3 serves a central role in the atherogenesis induced by oscillatory stress and disturbed blood flow. | |||
| Responsed Disease | Atherosclerosis [ICD-11: BD40.Z] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| In-vitro Model | MAEC | Normal | Mus musculus | CVCL_U411 |
| HUVEC-C | Normal | Homo sapiens | CVCL_2959 | |
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00708)
| In total 29 m6A sequence/site(s) in this target gene | |||
| mod ID: A2ISITE007703 | Click to Show/Hide the Full List | ||
| mod site | chr17:5512490-5512491:- | [2] | |
| Sequence | AAGAAGATTTAAAAGACAAGGGGCAAAGAAAAATGCAATGG | ||
| Transcript ID List | ENST00000544378.6; ENST00000613500.4; ENST00000634467.1; ENST00000262467.10; ENST00000574512.1 | ||
| External Link | RMBase: RNA-editing_site_54746 | ||
| mod ID: A2ISITE007704 | Click to Show/Hide the Full List | ||
| mod site | chr17:5512821-5512822:- | [3] | |
| Sequence | AGGGACTCCTGGTTGTCCTCACTGCTCATTATATGCTAATT | ||
| Transcript ID List | rmsk_4623556; ENST00000613500.4; ENST00000544378.6; ENST00000262467.10; ENST00000574512.1 | ||
| External Link | RMBase: RNA-editing_site_54747 | ||
| mod ID: A2ISITE007705 | Click to Show/Hide the Full List | ||
| mod site | chr17:5512847-5512848:- | [3] | |
| Sequence | CCCACCAAAGCCAAGATGGCAATGAAAGGGACTCCTGGTTG | ||
| Transcript ID List | ENST00000262467.10; ENST00000574512.1; ENST00000613500.4; rmsk_4623556; ENST00000544378.6 | ||
| External Link | RMBase: RNA-editing_site_54748 | ||
| mod ID: A2ISITE007706 | Click to Show/Hide the Full List | ||
| mod site | chr17:5512854-5512855:- | [3] | |
| Sequence | GCCAAAGCCCACCAAAGCCAAGATGGCAATGAAAGGGACTC | ||
| Transcript ID List | ENST00000262467.10; ENST00000574512.1; ENST00000544378.6; ENST00000613500.4; rmsk_4623556 | ||
| External Link | RMBase: RNA-editing_site_54749 | ||
| mod ID: A2ISITE007707 | Click to Show/Hide the Full List | ||
| mod site | chr17:5512882-5512883:- | [3] | |
| Sequence | CCACTGATAAGATGCGGTAAAGAAGCTGGCCAAAGCCCACC | ||
| Transcript ID List | ENST00000574512.1; ENST00000613500.4; ENST00000262467.10; ENST00000544378.6; rmsk_4623556 | ||
| External Link | RMBase: RNA-editing_site_54750 | ||
| mod ID: A2ISITE007708 | Click to Show/Hide the Full List | ||
| mod site | chr17:5512883-5512884:- | [3] | |
| Sequence | TCCACTGATAAGATGCGGTAAAGAAGCTGGCCAAAGCCCAC | ||
| Transcript ID List | rmsk_4623556; ENST00000574512.1; ENST00000262467.10; ENST00000613500.4; ENST00000544378.6 | ||
| External Link | RMBase: RNA-editing_site_54751 | ||
| mod ID: A2ISITE007709 | Click to Show/Hide the Full List | ||
| mod site | chr17:5512908-5512909:- | [3] | |
| Sequence | TTCACAAGATGCAGGTCACAAAGACTCCACTGATAAGATGC | ||
| Transcript ID List | ENST00000262467.10; ENST00000574512.1; ENST00000544378.6; ENST00000613500.4; rmsk_4623556 | ||
| External Link | RMBase: RNA-editing_site_54752 | ||
| mod ID: A2ISITE007710 | Click to Show/Hide the Full List | ||
| mod site | chr17:5512948-5512949:- | [3] | |
| Sequence | CTCTAAGCTGGGGGTGACACAGGAGGTGGGCAGGACTGGCT | ||
| Transcript ID List | ENST00000262467.10; ENST00000613500.4; ENST00000574512.1; ENST00000544378.6 | ||
| External Link | RMBase: RNA-editing_site_54753 | ||
| mod ID: A2ISITE007711 | Click to Show/Hide the Full List | ||
| mod site | chr17:5512963-5512964:- | [3] | |
| Sequence | TTACTGTATGCAGATCTCTAAGCTGGGGGTGACACAGGAGG | ||
| Transcript ID List | ENST00000262467.10; ENST00000574512.1; ENST00000544378.6; ENST00000613500.4 | ||
| External Link | RMBase: RNA-editing_site_54754 | ||
| mod ID: A2ISITE007712 | Click to Show/Hide the Full List | ||
| mod site | chr17:5512964-5512965:- | [2] | |
| Sequence | TTTACTGTATGCAGATCTCTAAGCTGGGGGTGACACAGGAG | ||
| Transcript ID List | ENST00000544378.6; ENST00000574512.1; ENST00000262467.10; ENST00000613500.4 | ||
| External Link | RMBase: RNA-editing_site_54755 | ||
| mod ID: A2ISITE007713 | Click to Show/Hide the Full List | ||
| mod site | chr17:5513747-5513748:- | [3] | |
| Sequence | ACCAGTCCTGCCAACCTCCTATCTTATCCCCACCTCAGACA | ||
| Transcript ID List | ENST00000544378.6; ENST00000262467.10; ENST00000574512.1; ENST00000613500.4 | ||
| External Link | RMBase: RNA-editing_site_54756 | ||
| mod ID: A2ISITE007714 | Click to Show/Hide the Full List | ||
| mod site | chr17:5513767-5513768:- | [3] | |
| Sequence | ATACGAGGAGTCTTTGTGAAACCAGTCCTGCCAACCTCCTA | ||
| Transcript ID List | ENST00000262467.10; ENST00000544378.6; ENST00000613500.4; ENST00000574512.1 | ||
| External Link | RMBase: RNA-editing_site_54757 | ||
| mod ID: A2ISITE007715 | Click to Show/Hide the Full List | ||
| mod site | chr17:5513802-5513803:- | [3] | |
| Sequence | TGATGGGTTTTGGCTTCTTTACCACATCCTGTTTTATACGA | ||
| Transcript ID List | ENST00000574512.1; ENST00000613500.4; ENST00000544378.6; ENST00000262467.10 | ||
| External Link | RMBase: RNA-editing_site_54758 | ||
| mod ID: A2ISITE007716 | Click to Show/Hide the Full List | ||
| mod site | chr17:5513820-5513821:- | [3] | |
| Sequence | TTCATCACCATCTTGGTTTGATGGGTTTTGGCTTCTTTACC | ||
| Transcript ID List | ENST00000544378.6; ENST00000262467.10; ENST00000613500.4; ENST00000574512.1 | ||
| External Link | RMBase: RNA-editing_site_54759 | ||
| mod ID: A2ISITE007717 | Click to Show/Hide the Full List | ||
| mod site | chr17:5513834-5513835:- | [3] | |
| Sequence | AACCGGAGGTCCCTTTCATCACCATCTTGGTTTGATGGGTT | ||
| Transcript ID List | ENST00000613500.4; ENST00000574512.1; ENST00000544378.6; ENST00000262467.10 | ||
| External Link | RMBase: RNA-editing_site_54760 | ||
| mod ID: A2ISITE007718 | Click to Show/Hide the Full List | ||
| mod site | chr17:5513853-5513854:- | [3] | |
| Sequence | TATAATGAGCAGCGAGGACAACCGGAGGTCCCTTTCATCAC | ||
| Transcript ID List | ENST00000262467.10; ENST00000544378.6; ENST00000574512.1; ENST00000613500.4 | ||
| External Link | RMBase: RNA-editing_site_54761 | ||
| mod ID: A2ISITE007719 | Click to Show/Hide the Full List | ||
| mod site | chr17:5513877-5513878:- | [2] | |
| Sequence | TATGCTAATGTATTAAAATTAGCATATAATGAGCAGCGAGG | ||
| Transcript ID List | ENST00000262467.10; ENST00000574512.1; ENST00000544378.6; ENST00000613500.4 | ||
| External Link | RMBase: RNA-editing_site_54762 | ||
| mod ID: A2ISITE007720 | Click to Show/Hide the Full List | ||
| mod site | chr17:5555929-5555930:- | [3] | |
| Sequence | AGCTAATTTTTTGTCGTCTTAGTAGAAACGGGGTTTCACCA | ||
| Transcript ID List | ENST00000613500.4; ENST00000571307.1; ENST00000345221.7; ENST00000619223.4; ENST00000262467.10; ENST00000617618.4; ENST00000354411.7; ENST00000544378.6; ENST00000577119.5; ENST00000572272.6; ENST00000571451.6; ENST00000269280.8 | ||
| External Link | RMBase: RNA-editing_site_54763 | ||
| mod ID: A2ISITE007721 | Click to Show/Hide the Full List | ||
| mod site | chr17:5561882-5561883:- | [3] | |
| Sequence | GCTGGCTGAGTTCAGACAGAAGCAGACTGCTAGCAGACATT | ||
| Transcript ID List | ENST00000571451.6; ENST00000345221.7; ENST00000613500.4; ENST00000571307.1; ENST00000617618.4; ENST00000269280.8; ENST00000262467.10; ENST00000544378.6; ENST00000619223.4; ENST00000354411.7; ENST00000577119.5; ENST00000572272.6 | ||
| External Link | RMBase: RNA-editing_site_54764 | ||
| mod ID: A2ISITE007722 | Click to Show/Hide the Full List | ||
| mod site | chr17:5586977-5586978:- | [2] | |
| Sequence | CACTGCAATCTCTGCCTCCCAGGTTCAAGCAGTTCTCCTGC | ||
| Transcript ID List | ENST00000572143.1; ENST00000576905.5 | ||
| External Link | RMBase: RNA-editing_site_54765 | ||
| mod ID: A2ISITE007723 | Click to Show/Hide the Full List | ||
| mod site | chr17:5606004-5606005:- | [2] | |
| Sequence | GTCTCAAACTCCTGGCCTCAAGTGATCTGCCCACCTCGGCC | ||
| Transcript ID List | ENST00000572143.1; ENST00000576905.5 | ||
| External Link | RMBase: RNA-editing_site_54766 | ||
| mod ID: A2ISITE007724 | Click to Show/Hide the Full List | ||
| mod site | chr17:5606113-5606114:- | [2] | |
| Sequence | CCTCGGCCTCCTTGGTAGCTAGGACTACAGGTGCCTGCCAC | ||
| Transcript ID List | ENST00000576905.5; ENST00000572143.1 | ||
| External Link | RMBase: RNA-editing_site_54767 | ||
| mod ID: A2ISITE007725 | Click to Show/Hide the Full List | ||
| mod site | chr17:5607458-5607459:- | [2] | |
| Sequence | CATGGTGGCGCATGCCTGTAATCCCAGCTACTGAAGAGGCT | ||
| Transcript ID List | rmsk_4623756; ENST00000576905.5; ENST00000572143.1 | ||
| External Link | RMBase: RNA-editing_site_54768 | ||
| mod ID: A2ISITE007726 | Click to Show/Hide the Full List | ||
| mod site | chr17:5607459-5607460:- | [2] | |
| Sequence | GCATGGTGGCGCATGCCTGTAATCCCAGCTACTGAAGAGGC | ||
| Transcript ID List | ENST00000572143.1; rmsk_4623756; ENST00000576905.5 | ||
| External Link | RMBase: RNA-editing_site_54769 | ||
| mod ID: A2ISITE007727 | Click to Show/Hide the Full List | ||
| mod site | chr17:5607551-5607552:- | [2] | |
| Sequence | GAGGCAGGCAGATCACCTGAAGTCAGGAGTTCGAGACCAGC | ||
| Transcript ID List | ENST00000576905.5; rmsk_4623756; ENST00000572143.1 | ||
| External Link | RMBase: RNA-editing_site_54770 | ||
| mod ID: A2ISITE007728 | Click to Show/Hide the Full List | ||
| mod site | chr17:5607830-5607831:- | [2] | |
| Sequence | TTCTGAGTAGCCAGGACCACAGACACATGCCACCATACCTG | ||
| Transcript ID List | ENST00000576905.5; ENST00000572143.1 | ||
| External Link | RMBase: RNA-editing_site_54771 | ||
| mod ID: A2ISITE007729 | Click to Show/Hide the Full List | ||
| mod site | chr17:5607832-5607833:- | [2] | |
| Sequence | CCTTCTGAGTAGCCAGGACCACAGACACATGCCACCATACC | ||
| Transcript ID List | ENST00000576905.5; ENST00000572143.1 | ||
| External Link | RMBase: RNA-editing_site_54772 | ||
| mod ID: A2ISITE007730 | Click to Show/Hide the Full List | ||
| mod site | chr17:5607838-5607839:- | [3] | |
| Sequence | CCTCAGCCTTCTGAGTAGCCAGGACCACAGACACATGCCAC | ||
| Transcript ID List | ENST00000576905.5; ENST00000572143.1 | ||
| External Link | RMBase: RNA-editing_site_54773 | ||
| mod ID: A2ISITE007731 | Click to Show/Hide the Full List | ||
| mod site | chr17:5607878-5607879:- | [2] | |
| Sequence | CACTGCAGCCTCAAACTCCTAGGCTTAAGCAATATTCCAAC | ||
| Transcript ID List | ENST00000576905.5; ENST00000572143.1 | ||
| External Link | RMBase: RNA-editing_site_54774 | ||
N6-methyladenosine (m6A)
| In total 122 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE029699 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499589-5499590:- | [4] | |
| Sequence | CAAAAGGCTTAATACCAGGAACCACCTTGGCAAGATATTTA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HepG2; HEK293T; H1A; H1B; hNPCs; fibroblasts; Huh7; Jurkat; CD4T; GSC-11; HEK293A-TOA; MSC; TIME; TREX; iSLK; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000568641.1; ENST00000574406.1; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344588 | ||
| mod ID: M6ASITE029700 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499612-5499613:- | [4] | |
| Sequence | TAGGGATTTGTCATCTTAAGACTCAAAAGGCTTAATACCAG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HepG2; HEK293T; H1A; H1B; hNPCs; fibroblasts; GM12878; LCLs; Huh7; Jurkat; CD4T; GSC-11; HEK293A-TOA; MSC; TIME; TREX; iSLK; endometrial; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000574406.1; ENST00000544378.6; ENST00000568641.1 | ||
| External Link | RMBase: m6A_site_344589 | ||
| mod ID: M6ASITE029702 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499645-5499646:- | [5] | |
| Sequence | ACAAGAGTTCGTGCGGGAAAACCACCTGATCCCTAGGGATT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HepG2; HEK293T; A549; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; Huh7; Jurkat; CD4T; GSC-11; HEK293A-TOA; MSC; TIME; TREX; iSLK; endometrial; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000574406.1; ENST00000568641.1; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344590 | ||
| mod ID: M6ASITE029703 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499665-5499666:- | [6] | |
| Sequence | GGCTGAGGGATTTCTCATTGACAAGAGTTCGTGCGGGAAAA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | HEK293T; hESC-HEK293T | ||
| Seq Type List | DART-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000568641.1; ENST00000574406.1; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344591 | ||
| mod ID: M6ASITE029704 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499693-5499694:- | [7] | |
| Sequence | CTGGAGCTGTGAGACCCAAGACAAAAGGGGCTGAGGGATTT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; Huh7; Jurkat; CD4T; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000574406.1; ENST00000568641.1 | ||
| External Link | RMBase: m6A_site_344592 | ||
| mod ID: M6ASITE029705 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499700-5499701:- | [7] | |
| Sequence | CTCACTTCTGGAGCTGTGAGACCCAAGACAAAAGGGGCTGA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; Huh7; Jurkat; CD4T; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000568641.1; ENST00000544378.6; ENST00000574406.1 | ||
| External Link | RMBase: m6A_site_344593 | ||
| mod ID: M6ASITE029706 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499748-5499749:- | [7] | |
| Sequence | CGGGAGGGCTGTCCTTGGGGACTCTAGGATGGCTTCGTTCT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; Huh7; Jurkat; CD4T; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000574406.1; ENST00000544378.6; ENST00000568641.1 | ||
| External Link | RMBase: m6A_site_344594 | ||
| mod ID: M6ASITE029707 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499794-5499795:- | [5] | |
| Sequence | GGCGCACTCCTGAGGATGAGACTCTGGGGGCCCTAGCCGGG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HepG2; HEK293T; HeLa; H1A; H1B; hNPCs; hESCs; fibroblasts; A549; GM12878; LCLs; Jurkat; CD4T; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000574406.1; ENST00000544378.6; ENST00000568641.1 | ||
| External Link | RMBase: m6A_site_344595 | ||
| mod ID: M6ASITE029708 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499841-5499842:- | [5] | |
| Sequence | CGCGGGACCTGTGGCTGCAGACCCCGCCGGCACGCAGGCCC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HepG2; HEK293T; HeLa; H1A; H1B; hNPCs; fibroblasts; A549; LCLs; Jurkat; CD4T; GSC-11; HEK293A-TOA; MSC; TIME; TREX; iSLK; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000568641.1; ENST00000574406.1; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344596 | ||
| mod ID: M6ASITE029709 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499855-5499856:- | [4] | |
| Sequence | AAGTGTCCGGGACTCGCGGGACCTGTGGCTGCAGACCCCGC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HepG2; HEK293T; H1A; H1B; hNPCs; fibroblasts; A549; Jurkat; CD4T; GSC-11; HEK293A-TOA; TIME; TREX; iSLK; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000574406.1; ENST00000568641.1 | ||
| External Link | RMBase: m6A_site_344597 | ||
| mod ID: M6ASITE029710 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499864-5499865:- | [4] | |
| Sequence | ACGTCCTGGAAGTGTCCGGGACTCGCGGGACCTGTGGCTGC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HepG2; HEK293T; H1A; H1B; hNPCs; fibroblasts; A549; Jurkat; CD4T; GSC-11; HEK293A-TOA; TIME; TREX; iSLK; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000574406.1; ENST00000568641.1 | ||
| External Link | RMBase: m6A_site_344598 | ||
| mod ID: M6ASITE029711 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499887-5499888:- | [4] | |
| Sequence | AGGAGCTGTCTCACCAGGAGACCACGTCCTGGAAGTGTCCG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HepG2; HEK293T; U2OS; H1A; H1B; fibroblasts; Jurkat; CD4T; GSC-11; HEK293A-TOA; TREX; iSLK; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000574406.1; ENST00000544378.6; ENST00000568641.1 | ||
| External Link | RMBase: m6A_site_344599 | ||
| mod ID: M6ASITE029713 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499919-5499920:- | [4] | |
| Sequence | CGCTGCCCGGAGGAGTCGAGACTGGTACCCGGAGGAGCTGT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HepG2; HEK293T; U2OS; H1A; H1B; Huh7; Jurkat; CD4T; GSC-11; HEK293A-TOA; endometrial; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000568641.1; ENST00000574406.1 | ||
| External Link | RMBase: m6A_site_344600 | ||
| mod ID: M6ASITE029714 | Click to Show/Hide the Full List | ||
| mod site | chr17:5500034-5500035:- | [4] | |
| Sequence | AGGCGAAGCTGGAGGGCGGGACTCGGTAAGTGGCGTTCGTC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HepG2; HEK293T; Jurkat; CD4T; GSC-11; HEK293A-TOA; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000574406.1; ENST00000568641.1 | ||
| External Link | RMBase: m6A_site_344601 | ||
| mod ID: M6ASITE029715 | Click to Show/Hide the Full List | ||
| mod site | chr17:5500069-5500070:- | [4] | |
| Sequence | GTTGGCGCGGCCGAGAGAGGACAAGAGCGCGCAGCAGGCGA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HepG2; HEK293T; Jurkat; CD4T; GSC-11; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000568641.1; ENST00000574406.1; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344602 | ||
| mod ID: M6ASITE029716 | Click to Show/Hide the Full List | ||
| mod site | chr17:5500514-5500515:- | [8] | |
| Sequence | TCGGCATTTTGTGTTTTAGAACAGCGCTGCACCCCCTTCAT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HEK293A-TOA | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000574406.1; ENST00000544378.6; ENST00000568641.1 | ||
| External Link | RMBase: m6A_site_344603 | ||
| mod ID: M6ASITE029717 | Click to Show/Hide the Full List | ||
| mod site | chr17:5500565-5500566:- | [9] | |
| Sequence | CGGGACGGGGTGGGCGCAGGACCGAGCGGGGAGGGAAAGGG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | CD8T; HEK293A-TOA; TIME | ||
| Seq Type List | m6A-CLIP/IP; m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000568641.1; ENST00000574406.1 | ||
| External Link | RMBase: m6A_site_344604 | ||
| mod ID: M6ASITE029718 | Click to Show/Hide the Full List | ||
| mod site | chr17:5500858-5500859:- | [4] | |
| Sequence | TGACAAGCCTCCCCCGGAAGACCCTCCCGCTGCCCGCGGGC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HepG2; Jurkat; CD4T; GSC-11; HEK293T; HEK293A-TOA; TREX; iSLK; TIME; MSC; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000574406.1; ENST00000544378.6; ENST00000568641.1 | ||
| External Link | RMBase: m6A_site_344605 | ||
| mod ID: M6ASITE029719 | Click to Show/Hide the Full List | ||
| mod site | chr17:5500900-5500901:- | [4] | |
| Sequence | TCTCGAGCAGATTCGCCCGGACCCCGAGTCCGAAGGCCTGT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HepG2; Jurkat; CD4T; GSC-11; HEK293T; HEK293A-TOA; TREX; iSLK; TIME; MSC; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000568641.1; ENST00000574406.1; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344606 | ||
| mod ID: M6ASITE029720 | Click to Show/Hide the Full List | ||
| mod site | chr17:5500929-5500930:- | [4] | |
| Sequence | GACGGGCTCTTTCGGCGCAGACCCTGGGGTCTCGAGCAGAT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HepG2; Jurkat; CD4T; GSC-11; HEK293T; HEK293A-TOA; TREX; iSLK; MSC; TIME; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000574406.1; ENST00000568641.1; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344607 | ||
| mod ID: M6ASITE029721 | Click to Show/Hide the Full List | ||
| mod site | chr17:5500988-5500989:- | [10] | |
| Sequence | CCCACTGCGCTCTGACCCAGACCCGGCTGACCCACCTACCC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | MT4; Jurkat; CD4T; GSC-11; HEK293T; HEK293A-TOA; MSC; TREX; TIME; endometrial; GSCs | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000568641.1; ENST00000574406.1 | ||
| External Link | RMBase: m6A_site_344608 | ||
| mod ID: M6ASITE029722 | Click to Show/Hide the Full List | ||
| mod site | chr17:5501086-5501087:- | [11] | |
| Sequence | TCAGCTGAGAGGACCGGCGGACCCTGCAGAGGCCCCCTGCC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | Jurkat; CD4T; GSC-11; MSC; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000574406.1; ENST00000568641.1 | ||
| External Link | RMBase: m6A_site_344609 | ||
| mod ID: M6ASITE029724 | Click to Show/Hide the Full List | ||
| mod site | chr17:5501094-5501095:- | [11] | |
| Sequence | GCGGCGCCTCAGCTGAGAGGACCGGCGGACCCTGCAGAGGC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | Jurkat; CD4T; GSC-11; MSC; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000568641.1; ENST00000574406.1 | ||
| External Link | RMBase: m6A_site_344610 | ||
| mod ID: M6ASITE029725 | Click to Show/Hide the Full List | ||
| mod site | chr17:5501402-5501403:- | [4] | |
| Sequence | CATTAAGATTAAAATTTAAAACAGACCACACAATAAATATT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HepG2; HEK293T; U2OS; Huh7; Jurkat; CD4T; GSC-11; HEK293A-TOA; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000544378.6; ENST00000574406.1 | ||
| External Link | RMBase: m6A_site_344611 | ||
| mod ID: M6ASITE029726 | Click to Show/Hide the Full List | ||
| mod site | chr17:5501522-5501523:- | [12] | |
| Sequence | TTGAGCTGTATTATCTCTGGACCTTGGGATTGTGGGAGGCT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | GM12878; LCLs; CD8T; Huh7; CD4T; MSC; TIME; TREX; endometrial; NB4 | ||
| Seq Type List | m6A-seq; m6A-CLIP/IP; MeRIP-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000574406.1; ENST00000544378.6; rmsk_4623523 | ||
| External Link | RMBase: m6A_site_344612 | ||
| mod ID: M6ASITE029727 | Click to Show/Hide the Full List | ||
| mod site | chr17:5501584-5501585:- | [13] | |
| Sequence | ATACAGATGCAGATGGAGAGACAGAGAAAAAAAAGGAAGAA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | CD34; GM12878; LCLs; Huh7; CD4T; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000574406.1; rmsk_4623523; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344613 | ||
| mod ID: M6ASITE029728 | Click to Show/Hide the Full List | ||
| mod site | chr17:5501617-5501618:- | [13] | |
| Sequence | GTGAAGAGCAAGAAGAGAAAACAGGTTGTACACATACAGAT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | CD34; GM12878; LCLs; Huh7; CD4T; TIME; TREX; MSC; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000574406.1; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344614 | ||
| mod ID: M6ASITE029729 | Click to Show/Hide the Full List | ||
| mod site | chr17:5501742-5501743:- | [13] | |
| Sequence | TGACAAGGAGGCCTGAGTAGACCGCAGGTGGGTCTGAGAAA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | CD34; U2OS; LCLs; Huh7; CD4T; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000574406.1; ENST00000262467.10 | ||
| External Link | RMBase: m6A_site_344615 | ||
| mod ID: M6ASITE029730 | Click to Show/Hide the Full List | ||
| mod site | chr17:5501847-5501848:- | [13] | |
| Sequence | GAACACCAGCCAGCCGTGGAACCTCAGGTGCAACAGAGACG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | CD34; MT4; Huh7; CD4T; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000613500.4; ENST00000574406.1; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344616 | ||
| mod ID: M6ASITE029731 | Click to Show/Hide the Full List | ||
| mod site | chr17:5501865-5501866:- | [13] | |
| Sequence | TCCCTCCATTCCAGGAAGGAACACCAGCCAGCCGTGGAACC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | CD34; CD4T | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000574406.1; ENST00000544378.6; ENST00000613500.4; ENST00000262467.10 | ||
| External Link | RMBase: m6A_site_344617 | ||
| mod ID: M6ASITE029732 | Click to Show/Hide the Full List | ||
| mod site | chr17:5501911-5501912:- | [11] | |
| Sequence | GACCTCTCCAATCTACTGGGACTCAAATAAATCCAAGTGAT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | CD4T | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000574406.1; ENST00000262467.10; ENST00000613500.4; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344618 | ||
| mod ID: M6ASITE029733 | Click to Show/Hide the Full List | ||
| mod site | chr17:5502648-5502649:- | [14] | |
| Sequence | CATTCTTTCACTCCACCTAAACCATCCTTCCATTCCACCCT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000613500.4; ENST00000574512.1; ENST00000262467.10; ENST00000544378.6; ENST00000574406.1 | ||
| External Link | RMBase: m6A_site_344619 | ||
| mod ID: M6ASITE029735 | Click to Show/Hide the Full List | ||
| mod site | chr17:5503099-5503100:- | [13] | |
| Sequence | CCTTCCATTCCACCTTGAAAACCCTCCCATTGCACCCTACA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | CD34; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000574512.1; ENST00000613500.4; ENST00000262467.10; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344620 | ||
| mod ID: M6ASITE029736 | Click to Show/Hide the Full List | ||
| mod site | chr17:5503207-5503208:- | [14] | |
| Sequence | CCTTCCATTCCACCTTGAAAACCCTCTCATTGCACCCTACA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000613500.4; ENST00000262467.10; ENST00000574512.1 | ||
| External Link | RMBase: m6A_site_344621 | ||
| mod ID: M6ASITE029737 | Click to Show/Hide the Full List | ||
| mod site | chr17:5503329-5503330:- | [14] | |
| Sequence | AGACCCTCTTGGTTACTGAGACTTCCCCACTACCTACTCCC | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000574512.1; ENST00000544378.6; ENST00000613500.4; ENST00000262467.10 | ||
| External Link | RMBase: m6A_site_344622 | ||
| mod ID: M6ASITE029738 | Click to Show/Hide the Full List | ||
| mod site | chr17:5503347-5503348:- | [14] | |
| Sequence | TTTGCATGGTTCTGCCCGAGACCCTCTTGGTTACTGAGACT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000613500.4; ENST00000544378.6; ENST00000574512.1; ENST00000262467.10 | ||
| External Link | RMBase: m6A_site_344623 | ||
| mod ID: M6ASITE029739 | Click to Show/Hide the Full List | ||
| mod site | chr17:5505950-5505951:- | [14] | |
| Sequence | CACAAAGAGAAGAGGACAGAACAAAAGTAACCAAATCTATT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000574512.1; ENST00000544378.6; ENST00000262467.10; ENST00000613500.4 | ||
| External Link | RMBase: m6A_site_344624 | ||
| mod ID: M6ASITE029740 | Click to Show/Hide the Full List | ||
| mod site | chr17:5505955-5505956:- | [14] | |
| Sequence | AGCGGCACAAAGAGAAGAGGACAGAACAAAAGTAACCAAAT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000574512.1; ENST00000613500.4; ENST00000262467.10 | ||
| External Link | RMBase: m6A_site_344625 | ||
| mod ID: M6ASITE029741 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514213-5514214:- | [14] | |
| Sequence | TTTCCAGTTTTTACCAGAAAACCCCTATAAATTAAAAATTT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | rmsk_4623561; ENST00000572272.6; ENST00000544378.6; ENST00000571451.6; ENST00000269280.8; ENST00000613500.4; ENST00000574512.1; ENST00000262467.10 | ||
| External Link | RMBase: m6A_site_344626 | ||
| mod ID: M6ASITE029742 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514416-5514417:- | [7] | |
| Sequence | AGGAATAGGAGGGACATGGAACCATTTGCCTCTGGCTGTGT | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; fibroblasts; LCLs; Huh7; Jurkat; CD4T; peripheral-blood; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000571451.6; ENST00000269280.8; ENST00000544378.6; ENST00000572272.6; ENST00000613500.4; ENST00000574512.1 | ||
| External Link | RMBase: m6A_site_344627 | ||
| mod ID: M6ASITE029743 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514423-5514424:- | [7] | |
| Sequence | CGCAAAGAGGAATAGGAGGGACATGGAACCATTTGCCTCTG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; hESC-HEK293T; fibroblasts; LCLs; CD8T; Huh7; Jurkat; CD4T; peripheral-blood; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MAZTER-seq; m6A-CLIP/IP; MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000574512.1; ENST00000571451.6; ENST00000269280.8; ENST00000613500.4; ENST00000262467.10; ENST00000572272.6 | ||
| External Link | RMBase: m6A_site_344628 | ||
| mod ID: M6ASITE029744 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514508-5514509:- | [13] | |
| Sequence | AGCTGGCAAGACCCCTGCAGACCTCATAGAGCCTCATCTGG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | CD34; Huh7; CD4T; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000572272.6; ENST00000571451.6; ENST00000544378.6; ENST00000613500.4; ENST00000269280.8; ENST00000574512.1 | ||
| External Link | RMBase: m6A_site_344629 | ||
| mod ID: M6ASITE029746 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514518-5514519:- | [13] | |
| Sequence | GTCCATCTGGAGCTGGCAAGACCCCTGCAGACCTCATAGAG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | CD34; Huh7; CD4T; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000571451.6; ENST00000262467.10; ENST00000544378.6; ENST00000572272.6; ENST00000574512.1; ENST00000613500.4; ENST00000269280.8 | ||
| External Link | RMBase: m6A_site_344630 | ||
| mod ID: M6ASITE029747 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514631-5514632:- | [13] | |
| Sequence | TCCAGCACTAAAGTAATGGAACTTTGATGATGCCTTTGCTG | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | CD34; GM12878; Huh7; CD4T; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000613500.4; ENST00000544378.6; ENST00000262467.10; ENST00000574512.1; ENST00000269280.8; ENST00000572272.6; ENST00000571451.6 | ||
| External Link | RMBase: m6A_site_344631 | ||
| mod ID: M6ASITE029748 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514674-5514675:- | [12] | |
| Sequence | CTCAGTTTCTTTCTCTGCAAACAAGTTGCCATCTGGTTTGC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | GM12878; Huh7; CD4T; peripheral-blood; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000269280.8; ENST00000574512.1; ENST00000572272.6; ENST00000613500.4; ENST00000544378.6; ENST00000262467.10; ENST00000571451.6 | ||
| External Link | RMBase: m6A_site_344632 | ||
| mod ID: M6ASITE029749 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514774-5514775:- | [7] | |
| Sequence | GAGAAGGGCAGCAAAAAGGGACTCCTGCCACTCAGCAGCTG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; U2OS; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000572272.6; ENST00000262467.10; ENST00000613500.4; ENST00000577119.5; ENST00000544378.6; ENST00000345221.7; ENST00000354411.7; ENST00000571451.6; ENST00000574512.1; ENST00000617618.4; ENST00000269280.8; ENST00000619223.4 | ||
| External Link | RMBase: m6A_site_344633 | ||
| mod ID: M6ASITE029750 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514801-5514802:- | [7] | |
| Sequence | CATCCTCACCTCATTATGGAACTCTGGGAGAAGGGCAGCAA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; U2OS; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000617618.4; ENST00000574512.1; ENST00000345221.7; ENST00000269280.8; ENST00000577119.5; ENST00000354411.7; ENST00000544378.6; ENST00000571451.6; ENST00000572272.6; ENST00000613500.4; ENST00000619223.4 | ||
| External Link | RMBase: m6A_site_344634 | ||
| mod ID: M6ASITE029751 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514824-5514825:- | [7] | |
| Sequence | TCTACCAAGCCCTGAAGGAGACCCATCCTCACCTCATTATG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; U2OS; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000577119.5; ENST00000345221.7; ENST00000262467.10; ENST00000574512.1; ENST00000613500.4; ENST00000269280.8; ENST00000571451.6; ENST00000572272.6; ENST00000354411.7; ENST00000544378.6; ENST00000619223.4; ENST00000617618.4 | ||
| External Link | RMBase: m6A_site_344635 | ||
| mod ID: M6ASITE029752 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514846-5514847:- | [7] | |
| Sequence | GACCGGAAGTGCAAAGATGGACTCTACCAAGCCCTGAAGGA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; U2OS; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000613500.4; ENST00000617618.4; ENST00000619223.4; ENST00000345221.7; ENST00000577119.5; ENST00000574512.1; ENST00000572272.6; ENST00000544378.6; ENST00000262467.10; ENST00000571451.6; ENST00000269280.8; ENST00000354411.7 | ||
| External Link | RMBase: m6A_site_344636 | ||
| mod ID: M6ASITE029753 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514865-5514866:- | [7] | |
| Sequence | CAGCTTGAGCCAGTCCTGGGACCGGAAGTGCAAAGATGGAC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; U2OS; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000574512.1; ENST00000577119.5; ENST00000613500.4; ENST00000572272.6; ENST00000269280.8; ENST00000544378.6; ENST00000571451.6; ENST00000354411.7; ENST00000262467.10; ENST00000617618.4; ENST00000619223.4; ENST00000345221.7 | ||
| External Link | RMBase: m6A_site_344637 | ||
| mod ID: M6ASITE029754 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514916-5514917:- | [7] | |
| Sequence | CGAGAGGGTGCTGGCTGAGAACACGAGGCCCAGCCAGATGC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; U2OS; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000345221.7; ENST00000571451.6; ENST00000572272.6; ENST00000574512.1; ENST00000617618.4; ENST00000354411.7; ENST00000619223.4; ENST00000613500.4; ENST00000544378.6; ENST00000262467.10; ENST00000269280.8; ENST00000577119.5 | ||
| External Link | RMBase: m6A_site_344638 | ||
| mod ID: M6ASITE029755 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514960-5514961:- | [7] | |
| Sequence | GTCTTGGACAAACTGCATGGACAGGTGCTGAGCCAGGAGCA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; U2OS; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000577119.5; ENST00000613500.4; ENST00000269280.8; ENST00000354411.7; ENST00000544378.6; ENST00000617618.4; ENST00000572272.6; ENST00000574512.1; ENST00000345221.7; ENST00000571451.6; ENST00000262467.10; ENST00000619223.4 | ||
| External Link | RMBase: m6A_site_344639 | ||
| mod ID: M6ASITE029757 | Click to Show/Hide the Full List | ||
| mod site | chr17:5514973-5514974:- | [7] | |
| Sequence | ATCGGTGGAGGTTGTCTTGGACAAACTGCATGGACAGGTGC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; U2OS; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000345221.7; ENST00000574512.1; ENST00000262467.10; ENST00000619223.4; ENST00000269280.8; ENST00000571451.6; ENST00000572272.6; ENST00000544378.6; ENST00000617618.4; ENST00000613500.4; ENST00000354411.7; ENST00000577119.5 | ||
| External Link | RMBase: m6A_site_344640 | ||
| mod ID: M6ASITE029758 | Click to Show/Hide the Full List | ||
| mod site | chr17:5515027-5515028:- | [7] | |
| Sequence | GCAGTTGCTGCACTTTGTGGACCAGTATCGAGAGCAGCTGA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; U2OS; fibroblasts; GM12878; LCLs; Huh7; CD4T; MSC; TIME; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000619223.4; ENST00000577119.5; ENST00000544378.6; ENST00000345221.7; ENST00000617618.4; ENST00000574512.1; ENST00000354411.7; ENST00000269280.8; ENST00000572272.6; ENST00000571451.6; ENST00000613500.4 | ||
| External Link | RMBase: m6A_site_344641 | ||
| mod ID: M6ASITE029759 | Click to Show/Hide the Full List | ||
| mod site | chr17:5517749-5517750:- | [13] | |
| Sequence | GTGTGGGAGGCCTTGGTGAAACCAGGTAAATCCAGGACAAT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | CD34; Huh7; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000572272.6; ENST00000262467.10; ENST00000617618.4; ENST00000619223.4; ENST00000345221.7; ENST00000354411.7; ENST00000571451.6; ENST00000574512.1; ENST00000269280.8; ENST00000544378.6; ENST00000577119.5; ENST00000613500.4 | ||
| External Link | RMBase: m6A_site_344642 | ||
| mod ID: M6ASITE029760 | Click to Show/Hide the Full List | ||
| mod site | chr17:5517775-5517776:- | [13] | |
| Sequence | TGAAAGACAAGAAAGATGAGACTCTGGTGTGGGAGGCCTTG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | CD34; Huh7; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000619223.4; ENST00000345221.7; ENST00000574512.1; ENST00000617618.4; ENST00000571451.6; ENST00000577119.5; ENST00000572272.6; ENST00000613500.4; ENST00000269280.8; ENST00000544378.6; ENST00000354411.7; ENST00000262467.10 | ||
| External Link | RMBase: m6A_site_344643 | ||
| mod ID: M6ASITE029761 | Click to Show/Hide the Full List | ||
| mod site | chr17:5517789-5517790:- | [13] | |
| Sequence | GATCAGGCTGCAAGTGAAAGACAAGAAAGATGAGACTCTGG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | CD34; Huh7; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000572272.6; ENST00000354411.7; ENST00000269280.8; ENST00000345221.7; ENST00000571451.6; ENST00000574512.1; ENST00000613500.4; ENST00000619223.4; ENST00000544378.6; ENST00000617618.4; ENST00000577119.5 | ||
| External Link | RMBase: m6A_site_344644 | ||
| mod ID: M6ASITE029762 | Click to Show/Hide the Full List | ||
| mod site | chr17:5517852-5517853:- | [13] | |
| Sequence | CTATCGAAGCCCTGGAGAAGACCAGCTGTTCTCGGAGTTCT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | CD34; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000269280.8; ENST00000572272.6; ENST00000262467.10; ENST00000345221.7; ENST00000574512.1; ENST00000577119.5; ENST00000619223.4; ENST00000354411.7; ENST00000613500.4; ENST00000617618.4; ENST00000544378.6; ENST00000571451.6 | ||
| External Link | RMBase: m6A_site_344645 | ||
| mod ID: M6ASITE029763 | Click to Show/Hide the Full List | ||
| mod site | chr17:5517884-5517885:- | [13] | |
| Sequence | AACTCTTTGCCTCTTCAGGAACTGGAGCTCTGCTATCGAAG | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000269280.8; ENST00000574512.1; ENST00000345221.7; ENST00000619223.4; ENST00000571451.6; ENST00000613500.4; ENST00000354411.7; ENST00000617618.4; ENST00000577119.5; ENST00000572272.6; ENST00000262467.10; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344646 | ||
| mod ID: M6ASITE029764 | Click to Show/Hide the Full List | ||
| mod site | chr17:5517919-5517920:- | [13] | |
| Sequence | AGACCTTCCATGCAAGATGAACAGGGTGGCAACTCAACTCT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000574512.1; ENST00000572272.6; ENST00000262467.10; ENST00000617618.4; ENST00000571451.6; ENST00000619223.4; ENST00000354411.7; ENST00000345221.7; ENST00000269280.8; ENST00000577119.5; ENST00000613500.4 | ||
| External Link | RMBase: m6A_site_344647 | ||
| mod ID: M6ASITE029765 | Click to Show/Hide the Full List | ||
| mod site | chr17:5524281-5524282:- | [9] | |
| Sequence | ACTGTCACAGGAAACAAGAGACAAAGTATCATGGACTACAA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000577119.5; ENST00000544378.6; ENST00000345221.7; ENST00000571451.6; ENST00000572272.6; ENST00000619223.4; ENST00000613500.4; ENST00000269280.8; ENST00000262467.10; ENST00000617618.4; ENST00000354411.7 | ||
| External Link | RMBase: m6A_site_344648 | ||
| mod ID: M6ASITE029766 | Click to Show/Hide the Full List | ||
| mod site | chr17:5533346-5533347:- | [10] | |
| Sequence | GTTGCTCAGGCTAATCTCAAACTCCTGGACGTGAGCAAGAT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | MT4; CD4T; endometrial | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000617618.4; ENST00000619223.4; ENST00000577119.5; ENST00000571451.6; ENST00000354411.7; ENST00000613500.4; ENST00000345221.7; ENST00000571307.1; ENST00000572272.6; ENST00000269280.8; ENST00000262467.10 | ||
| External Link | RMBase: m6A_site_344649 | ||
| mod ID: M6ASITE029768 | Click to Show/Hide the Full List | ||
| mod site | chr17:5533906-5533907:- | [13] | |
| Sequence | TCCTCACTCAAGCGGCAGAGACTCGGATCAGGTAGTTTGAA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | CD34; MT4; CD4T; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000571307.1; ENST00000577119.5; ENST00000345221.7; ENST00000269280.8; ENST00000613500.4; ENST00000617618.4; ENST00000354411.7; ENST00000544378.6; ENST00000571451.6; ENST00000262467.10; ENST00000572272.6; ENST00000619223.4 | ||
| External Link | RMBase: m6A_site_344650 | ||
| mod ID: M6ASITE029769 | Click to Show/Hide the Full List | ||
| mod site | chr17:5533984-5533985:- | [13] | |
| Sequence | AACAATTCTTTGTCCAGGAAACCAAGTGTGATGACCCCTAC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | CD34; MT4; CD4T; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000617618.4; ENST00000577119.5; ENST00000354411.7; ENST00000619223.4; ENST00000269280.8; ENST00000613500.4; ENST00000572272.6; ENST00000345221.7; ENST00000571451.6; ENST00000571307.1; ENST00000262467.10; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344651 | ||
| mod ID: M6ASITE029770 | Click to Show/Hide the Full List | ||
| mod site | chr17:5536876-5536877:- | [13] | |
| Sequence | AGGGCCCTGGAGCAGGAGAAACCTCAGCTGCTCATCTTCAG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | CD34; MT4; Huh7; CD4T; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000571307.1; ENST00000619223.4; ENST00000617618.4; ENST00000544378.6; ENST00000269280.8; ENST00000345221.7; ENST00000577119.5; ENST00000571451.6; ENST00000262467.10; ENST00000354411.7; ENST00000572272.6; ENST00000613500.4 | ||
| External Link | RMBase: m6A_site_344652 | ||
| mod ID: M6ASITE029771 | Click to Show/Hide the Full List | ||
| mod site | chr17:5536900-5536901:- | [13] | |
| Sequence | AGTGATGAGATGAGGCAGGAACTGAGGGCCCTGGAGCAGGA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | CD34; Huh7; CD4T; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000577119.5; ENST00000613500.4; ENST00000571307.1; ENST00000269280.8; ENST00000619223.4; ENST00000617618.4; ENST00000345221.7; ENST00000571451.6; ENST00000572272.6; ENST00000544378.6; ENST00000354411.7; ENST00000262467.10 | ||
| External Link | RMBase: m6A_site_344653 | ||
| mod ID: M6ASITE029772 | Click to Show/Hide the Full List | ||
| mod site | chr17:5536929-5536930:- | [13] | |
| Sequence | GTGCTTTCAGGCTGGACCAGACAACTCTGAGTGATGAGATG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | CD34; Huh7; CD4T; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000619223.4; ENST00000617618.4; ENST00000613500.4; ENST00000571451.6; ENST00000262467.10; ENST00000571307.1; ENST00000572272.6; ENST00000354411.7; ENST00000345221.7; ENST00000269280.8; ENST00000544378.6; ENST00000577119.5 | ||
| External Link | RMBase: m6A_site_344654 | ||
| mod ID: M6ASITE029773 | Click to Show/Hide the Full List | ||
| mod site | chr17:5536934-5536935:- | [13] | |
| Sequence | CCTTTGTGCTTTCAGGCTGGACCAGACAACTCTGAGTGATG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | CD34; Huh7; CD4T; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000617618.4; ENST00000571451.6; ENST00000619223.4; ENST00000345221.7; ENST00000572272.6; ENST00000571307.1; ENST00000262467.10; ENST00000577119.5; ENST00000269280.8; ENST00000544378.6; ENST00000354411.7; ENST00000613500.4 | ||
| External Link | RMBase: m6A_site_344655 | ||
| mod ID: M6ASITE029774 | Click to Show/Hide the Full List | ||
| mod site | chr17:5539428-5539429:- | [13] | |
| Sequence | CTCAGGCATCCTGCCTGCAAACTCATACGCCTGGGGTAAGG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | CD34; CD4T; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000354411.7; ENST00000345221.7; ENST00000572272.6; ENST00000577119.5; ENST00000262467.10; ENST00000269280.8; ENST00000613500.4; ENST00000619223.4; ENST00000571451.6; ENST00000617618.4; ENST00000571307.1 | ||
| External Link | RMBase: m6A_site_344656 | ||
| mod ID: M6ASITE029775 | Click to Show/Hide the Full List | ||
| mod site | chr17:5539489-5539490:- | [13] | |
| Sequence | GGAGCTAGACCTGCAGCAGAACAACCTGGATGACGTTGGCG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | CD34; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000619223.4; ENST00000577119.5; ENST00000571451.6; ENST00000354411.7; ENST00000345221.7; ENST00000262467.10; ENST00000269280.8; ENST00000572272.6; ENST00000613500.4; ENST00000617618.4; ENST00000571307.1 | ||
| External Link | RMBase: m6A_site_344657 | ||
| mod ID: M6ASITE029776 | Click to Show/Hide the Full List | ||
| mod site | chr17:5539501-5539502:- | [13] | |
| Sequence | CCCCAGCCTGAAGGAGCTAGACCTGCAGCAGAACAACCTGG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | CD34; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000617618.4; ENST00000345221.7; ENST00000619223.4; ENST00000577119.5; ENST00000571451.6; ENST00000354411.7; ENST00000571307.1; ENST00000572272.6; ENST00000613500.4; ENST00000544378.6; ENST00000269280.8 | ||
| External Link | RMBase: m6A_site_344658 | ||
| mod ID: M6ASITE029777 | Click to Show/Hide the Full List | ||
| mod site | chr17:5539546-5539547:- | [15] | |
| Sequence | CACGTCTGACTGCTGCCAGGACCTGGCCTCTGTGCTTAGTG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000577119.5; ENST00000619223.4; ENST00000571451.6; ENST00000269280.8; ENST00000345221.7; ENST00000544378.6; ENST00000571307.1; ENST00000617618.4; ENST00000613500.4; ENST00000354411.7; ENST00000262467.10; ENST00000572272.6 | ||
| External Link | RMBase: m6A_site_344659 | ||
| mod ID: M6ASITE029779 | Click to Show/Hide the Full List | ||
| mod site | chr17:5541885-5541886:- | [15] | |
| Sequence | CACCTTTGCCAGAGACTGAGACAGCCGAGCTGCAAGCTACA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000571451.6; ENST00000613500.4; ENST00000345221.7; ENST00000269280.8; ENST00000571307.1; ENST00000572272.6; ENST00000619223.4; ENST00000577119.5; ENST00000262467.10; ENST00000354411.7; ENST00000544378.6; ENST00000617618.4 | ||
| External Link | RMBase: m6A_site_344660 | ||
| mod ID: M6ASITE029780 | Click to Show/Hide the Full List | ||
| mod site | chr17:5541891-5541892:- | [15] | |
| Sequence | GCCAAACACCTTTGCCAGAGACTGAGACAGCCGAGCTGCAA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000571307.1; ENST00000269280.8; ENST00000613500.4; ENST00000345221.7; ENST00000262467.10; ENST00000571451.6; ENST00000617618.4; ENST00000577119.5; ENST00000619223.4; ENST00000544378.6; ENST00000572272.6; ENST00000354411.7 | ||
| External Link | RMBase: m6A_site_344661 | ||
| mod ID: M6ASITE029781 | Click to Show/Hide the Full List | ||
| mod site | chr17:5541906-5541907:- | [15] | |
| Sequence | CTCACGGATGCTGGAGCCAAACACCTTTGCCAGAGACTGAG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619223.4; ENST00000613500.4; ENST00000571451.6; ENST00000617618.4; ENST00000354411.7; ENST00000577119.5; ENST00000262467.10; ENST00000572272.6; ENST00000269280.8; ENST00000345221.7; ENST00000571307.1; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344662 | ||
| mod ID: M6ASITE029782 | Click to Show/Hide the Full List | ||
| mod site | chr17:5541943-5541944:- | [15] | |
| Sequence | CCAGACCCTGACCGAGCTGGACCTGAGCTTCAATGTGCTCA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000571307.1; ENST00000572272.6; ENST00000345221.7; ENST00000619223.4; ENST00000544378.6; ENST00000613500.4; ENST00000617618.4; ENST00000571451.6; ENST00000269280.8; ENST00000577119.5; ENST00000354411.7 | ||
| External Link | RMBase: m6A_site_344663 | ||
| mod ID: M6ASITE029783 | Click to Show/Hide the Full List | ||
| mod site | chr17:5541959-5541960:- | [13] | |
| Sequence | TTGGGCTGAGAGCCAACCAGACCCTGACCGAGCTGGACCTG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | CD34; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000571307.1; ENST00000577119.5; ENST00000544378.6; ENST00000619223.4; ENST00000613500.4; ENST00000262467.10; ENST00000269280.8; ENST00000617618.4; ENST00000572272.6; ENST00000345221.7; ENST00000354411.7; ENST00000571451.6 | ||
| External Link | RMBase: m6A_site_344664 | ||
| mod ID: M6ASITE029784 | Click to Show/Hide the Full List | ||
| mod site | chr17:5541988-5541989:- | [13] | |
| Sequence | CACAGCTGAGGACTGCAAGGACCTTGCCTTTGGGCTGAGAG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | CD34; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000613500.4; ENST00000617618.4; ENST00000571451.6; ENST00000345221.7; ENST00000571307.1; ENST00000572272.6; ENST00000354411.7; ENST00000269280.8; ENST00000262467.10; ENST00000577119.5; ENST00000619223.4 | ||
| External Link | RMBase: m6A_site_344665 | ||
| mod ID: M6ASITE029785 | Click to Show/Hide the Full List | ||
| mod site | chr17:5541997-5541998:- | [13] | |
| Sequence | CTGTGGCCTCACAGCTGAGGACTGCAAGGACCTTGCCTTTG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | CD34; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000571451.6; ENST00000262467.10; ENST00000619223.4; ENST00000572272.6; ENST00000617618.4; ENST00000345221.7; ENST00000577119.5; ENST00000354411.7; ENST00000613500.4; ENST00000269280.8; ENST00000571307.1; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344666 | ||
| mod ID: M6ASITE029786 | Click to Show/Hide the Full List | ||
| mod site | chr17:5553392-5553393:- | [13] | |
| Sequence | GCCCTCGCTGCCTCCTGGAGACCCTGCGGTGAGTCTGGCCT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000577119.5; ENST00000354411.7; ENST00000571451.6; ENST00000572272.6; ENST00000613500.4; ENST00000571307.1; ENST00000345221.7; ENST00000269280.8; ENST00000262467.10; ENST00000619223.4; ENST00000544378.6; ENST00000617618.4 | ||
| External Link | RMBase: m6A_site_344667 | ||
| mod ID: M6ASITE029787 | Click to Show/Hide the Full List | ||
| mod site | chr17:5553422-5553423:- | [13] | |
| Sequence | CAGTGAAGAGTCTTTGTAAGACCCTGAGACGCCCTCGCTGC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000571451.6; ENST00000544378.6; ENST00000262467.10; ENST00000345221.7; ENST00000619223.4; ENST00000571307.1; ENST00000613500.4; ENST00000572272.6; ENST00000269280.8; ENST00000354411.7; ENST00000617618.4; ENST00000577119.5 | ||
| External Link | RMBase: m6A_site_344668 | ||
| mod ID: M6ASITE029788 | Click to Show/Hide the Full List | ||
| mod site | chr17:5558456-5558457:- | [13] | |
| Sequence | AAATGGGCATGTGTGTAGAAACAGACATGGAGCTCTTAGTG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | CD34; GM12878; LCLs; Huh7; TIME; TREX; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000617618.4; ENST00000571451.6; ENST00000619223.4; ENST00000577119.5; ENST00000345221.7; ENST00000544378.6; ENST00000354411.7; ENST00000572272.6; ENST00000262467.10; ENST00000269280.8; ENST00000571307.1; ENST00000613500.4 | ||
| External Link | RMBase: m6A_site_344669 | ||
| mod ID: M6ASITE029790 | Click to Show/Hide the Full List | ||
| mod site | chr17:5558515-5558516:- | [7] | |
| Sequence | CTGCTTGTACGAGACTCGGAACAAAACGTTCCTGACACAAG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; fibroblasts; GM12878; LCLs; Huh7; peripheral-blood; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000571451.6; ENST00000617618.4; ENST00000354411.7; ENST00000262467.10; ENST00000572272.6; ENST00000577119.5; ENST00000619223.4; ENST00000269280.8; ENST00000571307.1; ENST00000544378.6; ENST00000613500.4; ENST00000345221.7 | ||
| External Link | RMBase: m6A_site_344670 | ||
| mod ID: M6ASITE029791 | Click to Show/Hide the Full List | ||
| mod site | chr17:5558522-5558523:- | [7] | |
| Sequence | CCCTCCACTGCTTGTACGAGACTCGGAACAAAACGTTCCTG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; fibroblasts; GM12878; LCLs; Huh7; peripheral-blood; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000354411.7; ENST00000262467.10; ENST00000613500.4; ENST00000571307.1; ENST00000345221.7; ENST00000572272.6; ENST00000617618.4; ENST00000577119.5; ENST00000571451.6; ENST00000619223.4; ENST00000544378.6; ENST00000269280.8 | ||
| External Link | RMBase: m6A_site_344671 | ||
| mod ID: M6ASITE029792 | Click to Show/Hide the Full List | ||
| mod site | chr17:5558599-5558600:- | [7] | |
| Sequence | CCGGCTGTCTCAGGGGAGGAACCTGATGCAGTGGGTCCCGT | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; GM12878; LCLs; Huh7; peripheral-blood; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000345221.7; ENST00000613500.4; ENST00000544378.6; ENST00000577119.5; ENST00000571451.6; ENST00000617618.4; ENST00000269280.8; ENST00000262467.10; ENST00000572272.6; ENST00000354411.7; ENST00000571307.1; ENST00000619223.4 | ||
| External Link | RMBase: m6A_site_344672 | ||
| mod ID: M6ASITE029793 | Click to Show/Hide the Full List | ||
| mod site | chr17:5558632-5558633:- | [7] | |
| Sequence | GGGGGAGAGAGAGATGGAGAACATCTTTCACTGCCGGCTGT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; GM12878; LCLs; Huh7; MSC; TIME; TREX; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000617618.4; ENST00000572272.6; ENST00000269280.8; ENST00000577119.5; ENST00000345221.7; ENST00000262467.10; ENST00000354411.7; ENST00000571451.6; ENST00000544378.6; ENST00000613500.4; ENST00000571307.1; ENST00000619223.4 | ||
| External Link | RMBase: m6A_site_344673 | ||
| mod ID: M6ASITE029794 | Click to Show/Hide the Full List | ||
| mod site | chr17:5558729-5558730:- | [9] | |
| Sequence | GCATCATAGATTTGGAAAAGACGCTAGAAGCATATGGAATA | ||
| Motif Score | 2.871321429 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000571451.6; ENST00000571307.1; ENST00000577119.5; ENST00000613500.4; ENST00000269280.8; ENST00000345221.7; ENST00000619223.4; ENST00000354411.7; ENST00000572272.6; ENST00000544378.6; ENST00000262467.10; ENST00000617618.4 | ||
| External Link | RMBase: m6A_site_344674 | ||
| mod ID: M6ASITE029795 | Click to Show/Hide the Full List | ||
| mod site | chr17:5558760-5558761:- | [13] | |
| Sequence | GATGAGAAGGGGAGAGGTAAACATTCTAATTGCATCATAGA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | CD34; GM12878; LCLs; Huh7; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000613500.4; ENST00000572272.6; ENST00000577119.5; ENST00000345221.7; ENST00000571307.1; ENST00000617618.4; ENST00000571451.6; ENST00000544378.6; ENST00000262467.10; ENST00000269280.8; ENST00000619223.4; ENST00000354411.7 | ||
| External Link | RMBase: m6A_site_344675 | ||
| mod ID: M6ASITE029796 | Click to Show/Hide the Full List | ||
| mod site | chr17:5558945-5558946:- | [7] | |
| Sequence | AGGGCATCTGGCAAAAAAAGACCCTTTTCAGTCCAGATGAC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; fibroblasts; GM12878; LCLs; H1299; Huh7; Jurkat; peripheral-blood; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000571451.6; ENST00000572272.6; ENST00000262467.10; ENST00000613500.4; ENST00000619223.4; ENST00000544378.6; ENST00000345221.7; ENST00000269280.8; ENST00000571307.1; ENST00000354411.7; ENST00000577119.5; ENST00000617618.4 | ||
| External Link | RMBase: m6A_site_344676 | ||
| mod ID: M6ASITE029797 | Click to Show/Hide the Full List | ||
| mod site | chr17:5558986-5558987:- | [7] | |
| Sequence | ATTGGGACCCCAGCTCAGAGACCTCTGCTCTCTGGCTGCTG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; GM12878; LCLs; H1299; Huh7; Jurkat; peripheral-blood; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000269280.8; ENST00000617618.4; ENST00000262467.10; ENST00000544378.6; ENST00000345221.7; ENST00000613500.4; ENST00000619223.4; ENST00000571307.1; ENST00000354411.7; ENST00000571451.6; ENST00000572272.6; ENST00000577119.5 | ||
| External Link | RMBase: m6A_site_344677 | ||
| mod ID: M6ASITE029798 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559000-5559001:- | [7] | |
| Sequence | CTCCAAGCTCAGCCATTGGGACCCCAGCTCAGAGACCTCTG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; GM12878; LCLs; Huh7; Jurkat; peripheral-blood; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000619223.4; ENST00000571451.6; ENST00000617618.4; ENST00000571307.1; ENST00000577119.5; ENST00000269280.8; ENST00000544378.6; ENST00000613500.4; ENST00000345221.7; ENST00000572272.6; ENST00000354411.7 | ||
| External Link | RMBase: m6A_site_344678 | ||
| mod ID: M6ASITE029799 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559059-5559060:- | [7] | |
| Sequence | AACTCACACTGACTTCCAAGACCACCACAACCCTCTGTCTA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; GM12878; Huh7; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000354411.7; ENST00000345221.7; ENST00000617618.4; ENST00000619223.4; ENST00000613500.4; ENST00000571307.1; ENST00000269280.8; ENST00000572272.6; ENST00000571451.6; ENST00000577119.5; ENST00000262467.10 | ||
| External Link | RMBase: m6A_site_344679 | ||
| mod ID: M6ASITE029801 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559078-5559079:- | [7] | |
| Sequence | CAGATGAAGCGGAAGGAAAAACTCACACTGACTTCCAAGAC | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; GM12878; Huh7; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000613500.4; ENST00000354411.7; ENST00000571307.1; ENST00000617618.4; ENST00000262467.10; ENST00000577119.5; ENST00000345221.7; ENST00000544378.6; ENST00000571451.6; ENST00000619223.4; ENST00000572272.6; ENST00000269280.8 | ||
| External Link | RMBase: m6A_site_344680 | ||
| mod ID: M6ASITE029802 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559166-5559167:- | [13] | |
| Sequence | CTTTAGGTTGGTCAAATCAAACAAAGAGCTCTGGGCCCTGT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | CD34; GM12878; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000354411.7; ENST00000269280.8; ENST00000577119.5; ENST00000571307.1; ENST00000262467.10; ENST00000544378.6; ENST00000571451.6; ENST00000613500.4; ENST00000572272.6; ENST00000617618.4; ENST00000345221.7; ENST00000619223.4 | ||
| External Link | RMBase: m6A_site_344681 | ||
| mod ID: M6ASITE029803 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559301-5559302:- | [13] | |
| Sequence | TCGGACCACAGCTCTGCAGAACCTCATTCCTTCTTTGGAGC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | CD34; Huh7; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000269280.8; ENST00000571307.1; ENST00000572272.6; ENST00000544378.6; ENST00000617618.4; ENST00000262467.10; ENST00000345221.7; ENST00000354411.7; ENST00000571451.6; ENST00000619223.4; ENST00000577119.5; ENST00000613500.4 | ||
| External Link | RMBase: m6A_site_344682 | ||
| mod ID: M6ASITE029804 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559317-5559318:- | [13] | |
| Sequence | CCTTCCTGATCACGGCTCGGACCACAGCTCTGCAGAACCTC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | CD34; Huh7; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000354411.7; ENST00000617618.4; ENST00000345221.7; ENST00000619223.4; ENST00000571451.6; ENST00000544378.6; ENST00000613500.4; ENST00000269280.8; ENST00000571307.1; ENST00000572272.6; ENST00000577119.5 | ||
| External Link | RMBase: m6A_site_344683 | ||
| mod ID: M6ASITE029805 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559356-5559357:- | [13] | |
| Sequence | TGGGCAGTTTGCTGGGGAAAACTATACTTCCCGAGGCATCC | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | CD34; Huh7; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000572272.6; ENST00000577119.5; ENST00000544378.6; ENST00000354411.7; ENST00000262467.10; ENST00000619223.4; ENST00000269280.8; ENST00000613500.4; ENST00000345221.7; ENST00000571451.6; ENST00000571307.1; ENST00000617618.4 | ||
| External Link | RMBase: m6A_site_344684 | ||
| mod ID: M6ASITE029806 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559507-5559508:- | [11] | |
| Sequence | GCCACTCCGGCTCCCATTAGACAGATCCTGTCTAGGCCAGA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | CD4T; peripheral-blood; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000613500.4; ENST00000269280.8; ENST00000345221.7; ENST00000571451.6; ENST00000572272.6; ENST00000619223.4; ENST00000617618.4; ENST00000577119.5; ENST00000571307.1; ENST00000262467.10; ENST00000354411.7 | ||
| External Link | RMBase: m6A_site_344685 | ||
| mod ID: M6ASITE029807 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559530-5559531:- | [11] | |
| Sequence | AGCTCATCGGAAAAGATGGGACAGCCACTCCGGCTCCCATT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | CD4T; peripheral-blood; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000617618.4; ENST00000572272.6; ENST00000577119.5; ENST00000571451.6; ENST00000613500.4; ENST00000262467.10; ENST00000571307.1; ENST00000345221.7; ENST00000354411.7; ENST00000619223.4; ENST00000269280.8 | ||
| External Link | RMBase: m6A_site_344686 | ||
| mod ID: M6ASITE029808 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559619-5559620:- | [13] | |
| Sequence | GAGAGGCCAGCTGTATGGGGACCGCTTCCAGCATGTCTTCT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | CD34; GM12878; LCLs; H1299; Huh7; CD4T; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000617618.4; ENST00000613500.4; ENST00000262467.10; ENST00000269280.8; ENST00000544378.6; ENST00000571307.1; ENST00000354411.7; ENST00000572272.6; ENST00000345221.7; ENST00000577119.5; ENST00000571451.6; ENST00000619223.4 | ||
| External Link | RMBase: m6A_site_344687 | ||
| mod ID: M6ASITE029809 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559717-5559718:- | [13] | |
| Sequence | CCAGGCCTGGATACCCAAGAACCTCGCATAGTCATACTGCA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | CD34; HeLa; fibroblasts; GM12878; LCLs; H1299; Huh7; Jurkat; CD4T; MSC; TIME; TREX; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000613500.4; ENST00000354411.7; ENST00000577119.5; ENST00000617618.4; ENST00000544378.6; ENST00000571307.1; ENST00000619223.4; ENST00000572272.6; ENST00000345221.7; ENST00000262467.10; ENST00000269280.8; ENST00000571451.6 | ||
| External Link | RMBase: m6A_site_344688 | ||
| mod ID: M6ASITE029810 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559748-5559749:- | [13] | |
| Sequence | ACATTTAATTGAGATCAGAGACTTATTTGGCCCAGGCCTGG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | CD34; HeLa; fibroblasts; GM12878; LCLs; H1299; Huh7; Jurkat; CD4T; MSC; TIME; TREX; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000617618.4; ENST00000571307.1; ENST00000262467.10; ENST00000345221.7; ENST00000577119.5; ENST00000619223.4; ENST00000571451.6; ENST00000354411.7; ENST00000572272.6; ENST00000544378.6; ENST00000613500.4; ENST00000269280.8 | ||
| External Link | RMBase: m6A_site_344689 | ||
| mod ID: M6ASITE029812 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559768-5559769:- | [13] | |
| Sequence | TATGTGGAGGAGAATCGAGGACATTTAATTGAGATCAGAGA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | CD34; HeLa; hESC-HEK293T; fibroblasts; GM12878; LCLs; CD8T; H1299; Huh7; Jurkat; CD4T; MSC; TIME; TREX; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000354411.7; ENST00000345221.7; ENST00000572272.6; ENST00000571307.1; ENST00000577119.5; ENST00000617618.4; ENST00000619223.4; ENST00000269280.8; ENST00000262467.10; ENST00000544378.6; ENST00000613500.4; ENST00000571451.6 | ||
| External Link | RMBase: m6A_site_344690 | ||
| mod ID: M6ASITE029813 | Click to Show/Hide the Full List | ||
| mod site | chr17:5559837-5559838:- | [13] | |
| Sequence | CAGCTGCTACTTCTACAAAGACCTCACCCCAGAAGCCAAGA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | CD34; fibroblasts; GM12878; LCLs; H1299; Huh7; Jurkat; CD4T; TIME; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000572272.6; ENST00000619223.4; ENST00000345221.7; ENST00000613500.4; ENST00000571307.1; ENST00000354411.7; ENST00000577119.5; ENST00000262467.10; ENST00000617618.4; ENST00000269280.8; ENST00000571451.6; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344691 | ||
| mod ID: M6ASITE029814 | Click to Show/Hide the Full List | ||
| mod site | chr17:5581900-5581901:- | [13] | |
| Sequence | GAGAGCAGGAGGCTCCTGGGACCCAATGGCCTCTGGATGAA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | CD34; LCLs; Huh7; CD4T; MSC; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000571307.1; ENST00000572272.6; ENST00000345221.7; ENST00000354411.7; ENST00000571451.6; ENST00000617618.4; ENST00000262467.10; ENST00000577119.5; ENST00000619223.4; ENST00000544378.6; ENST00000613500.4; ENST00000269280.8 | ||
| External Link | RMBase: m6A_site_344692 | ||
| mod ID: M6ASITE029815 | Click to Show/Hide the Full List | ||
| mod site | chr17:5582016-5582017:- | [13] | |
| Sequence | AGCTCTTCCAAGCTCCCCAGACCATGAGTCTCCAAGCCAGG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | CD34; MT4; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000262467.10; ENST00000577119.5; ENST00000269280.8; ENST00000613500.4; ENST00000619223.4; ENST00000571451.6; ENST00000617618.4; ENST00000345221.7; ENST00000354411.7; ENST00000571307.1; ENST00000572272.6 | ||
| External Link | RMBase: m6A_site_344693 | ||
| mod ID: M6ASITE029816 | Click to Show/Hide the Full List | ||
| mod site | chr17:5582703-5582704:- | [13] | |
| Sequence | TCAGAGAGAAGGGTTTTGAGACAGCTGCCTGACACATCTGG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | CD34; MT4; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000617618.4; ENST00000544378.6; ENST00000345221.7; ENST00000354411.7; ENST00000571451.6; ENST00000577119.5; ENST00000619223.4; ENST00000262467.10; ENST00000613500.4; ENST00000571307.1; ENST00000572272.6; ENST00000269280.8 | ||
| External Link | RMBase: m6A_site_344694 | ||
| mod ID: M6ASITE029817 | Click to Show/Hide the Full List | ||
| mod site | chr17:5582811-5582812:- | [10] | |
| Sequence | TTCCCCTACAGCCCAAGTGAACCCCACCTGGGGTCTCCCAG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | MT4; Huh7; endometrial; HEC-1-A | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000571451.6; ENST00000619223.4; ENST00000571307.1; ENST00000613500.4; ENST00000354411.7; ENST00000572272.6; ENST00000577119.5; ENST00000262467.10; ENST00000345221.7; ENST00000617618.4; ENST00000269280.8 | ||
| External Link | RMBase: m6A_site_344695 | ||
| mod ID: M6ASITE029818 | Click to Show/Hide the Full List | ||
| mod site | chr17:5583754-5583755:- | [15] | |
| Sequence | TGGGGAGCAGCGGGCCTGGGACCTAGCCCTCCATACCTGGG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000354411.7; ENST00000269280.8; ENST00000617618.4; ENST00000544378.6; ENST00000613500.4; ENST00000577119.5; ENST00000619223.4; ENST00000262467.10; ENST00000571307.1; ENST00000571451.6; ENST00000572272.6; ENST00000345221.7 | ||
| External Link | RMBase: m6A_site_344696 | ||
| mod ID: M6ASITE029819 | Click to Show/Hide the Full List | ||
| mod site | chr17:5583836-5583837:- | [13] | |
| Sequence | CCAGGAGCTCTTCGGGTGAGACACCCGCTCAGCCAGAGAAG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | CD34; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000269280.8; ENST00000571307.1; ENST00000613500.4; ENST00000262467.10; ENST00000577119.5; ENST00000354411.7; ENST00000617618.4; ENST00000345221.7; ENST00000544378.6; ENST00000571451.6; ENST00000572272.6; ENST00000619223.4 | ||
| External Link | RMBase: m6A_site_344697 | ||
| mod ID: M6ASITE029820 | Click to Show/Hide the Full List | ||
| mod site | chr17:5583962-5583963:- | [13] | |
| Sequence | GACACCCTCTTAACTCCGGGACAGAGATGGCTGGCGGAGCC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | CD34; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000345221.7; ENST00000571451.6; ENST00000619223.4; ENST00000576905.5; ENST00000544378.6; ENST00000613500.4; ENST00000617618.4; ENST00000269280.8; ENST00000571307.1; ENST00000262467.10; ENST00000572272.6 | ||
| External Link | RMBase: m6A_site_344698 | ||
| mod ID: M6ASITE029821 | Click to Show/Hide the Full List | ||
| mod site | chr17:5583981-5583982:- | [13] | |
| Sequence | CACCTGGGAACATCCCCCAGACACCCTCTTAACTCCGGGAC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | CD34; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619223.4; ENST00000262467.10; ENST00000613500.4; ENST00000544378.6; ENST00000269280.8; ENST00000571451.6; ENST00000617618.4; ENST00000572272.6; ENST00000576905.5; ENST00000345221.7; ENST00000571307.1 | ||
| External Link | RMBase: m6A_site_344699 | ||
| mod ID: M6ASITE029823 | Click to Show/Hide the Full List | ||
| mod site | chr17:5583992-5583993:- | [13] | |
| Sequence | GATACCCTCACCACCTGGGAACATCCCCCAGACACCCTCTT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | CD34; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000262467.10; ENST00000572272.6; ENST00000345221.7; ENST00000269280.8; ENST00000571451.6; ENST00000617618.4; ENST00000571307.1; ENST00000613500.4; ENST00000619223.4; ENST00000544378.6; ENST00000576905.5 | ||
| External Link | RMBase: m6A_site_344700 | ||
| mod ID: M6ASITE029824 | Click to Show/Hide the Full List | ||
| mod site | chr17:5584035-5584036:- | [7] | |
| Sequence | GGTAAGAGCCAAGGCAAAGGACAGCACTGTTCTCTGCCTGC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000572272.6; ENST00000619223.4; ENST00000571451.6; ENST00000269280.8; ENST00000576905.5; ENST00000613500.4; ENST00000262467.10; ENST00000571307.1; ENST00000617618.4; ENST00000572143.1; ENST00000345221.7; ENST00000544378.6 | ||
| External Link | RMBase: m6A_site_344701 | ||
| mod ID: M6ASITE029825 | Click to Show/Hide the Full List | ||
| mod site | chr17:5584057-5584058:- | [9] | |
| Sequence | TTCCGAATGCTGAATAAGAGACGGTAAGAGCCAAGGCAAAG | ||
| Motif Score | 2.871321429 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000345221.7; ENST00000572272.6; ENST00000571307.1; ENST00000619223.4; ENST00000576905.5; ENST00000544378.6; ENST00000617618.4; ENST00000572143.1; ENST00000613500.4; ENST00000269280.8; ENST00000262467.10; ENST00000571451.6 | ||
| External Link | RMBase: m6A_site_344702 | ||
| mod ID: M6ASITE029826 | Click to Show/Hide the Full List | ||
| mod site | chr17:5584083-5584084:- | [7] | |
| Sequence | GGTGCCCTGGGATTTATAAAACTGGGTTCCGAATGCTGAAT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; CD8T; Huh7 | ||
| Seq Type List | m6A-seq; m6A-CLIP/IP; MeRIP-seq | ||
| Transcript ID List | ENST00000572143.1; ENST00000619223.4; ENST00000617618.4; ENST00000544378.6; ENST00000269280.8; ENST00000571451.6; ENST00000345221.7; ENST00000571307.1; ENST00000572272.6; ENST00000576905.5; ENST00000262467.10; ENST00000613500.4 | ||
| External Link | RMBase: m6A_site_344703 | ||
| mod ID: M6ASITE029827 | Click to Show/Hide the Full List | ||
| mod site | chr17:5584206-5584207:- | [14] | |
| Sequence | GCCCTGGACCCCATCCCAGGACCTCCCTATCAGCTGACTTC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | Huh7; HEC-1-A | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000544378.6; ENST00000613500.4; ENST00000576905.5; ENST00000571307.1; ENST00000269280.8; ENST00000345221.7; ENST00000617618.4; ENST00000262467.10; ENST00000572272.6; ENST00000619223.4; ENST00000571451.6; ENST00000572143.1 | ||
| External Link | RMBase: m6A_site_344704 | ||
| mod ID: M6ASITE029828 | Click to Show/Hide the Full List | ||
| mod site | chr17:5584219-5584220:- | [14] | |
| Sequence | CCCTCCCTGCCTGGCCCTGGACCCCATCCCAGGACCTCCCT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | Huh7; endometrial; HEC-1-A | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000345221.7; ENST00000544378.6; ENST00000572143.1; ENST00000617618.4; ENST00000571451.6; ENST00000262467.10; ENST00000619223.4; ENST00000571307.1; ENST00000572272.6; ENST00000269280.8; ENST00000613500.4; ENST00000576905.5 | ||
| External Link | RMBase: m6A_site_344705 | ||
| mod ID: M6ASITE029829 | Click to Show/Hide the Full List | ||
| mod site | chr17:5584294-5584295:- | [13] | |
| Sequence | AAGAATCTGAGGAACACAGAACAGTGAGCGTTGCCCACACC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | CD34; Huh7; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000571307.1; ENST00000572143.1; ENST00000572272.6; ENST00000544378.6; ENST00000269280.8; ENST00000619223.4; ENST00000617618.4; ENST00000613500.4; ENST00000345221.7; ENST00000571451.6; ENST00000576905.5; ENST00000262467.10 | ||
| External Link | RMBase: m6A_site_344706 | ||
| mod ID: M6ASITE029830 | Click to Show/Hide the Full List | ||
| mod site | chr17:5584301-5584302:- | [13] | |
| Sequence | GAGAGGGAAGAATCTGAGGAACACAGAACAGTGAGCGTTGC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | CD34; Huh7; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000619223.4; ENST00000544378.6; ENST00000572143.1; ENST00000572272.6; ENST00000571307.1; ENST00000576905.5; ENST00000617618.4; ENST00000269280.8; ENST00000571451.6; ENST00000262467.10; ENST00000613500.4; ENST00000345221.7 | ||
| External Link | RMBase: m6A_site_344707 | ||
| mod ID: M6ASITE029831 | Click to Show/Hide the Full List | ||
| mod site | chr17:5584327-5584328:- | [13] | |
| Sequence | GTTGATCTCAGGAGTCCAGGACCCAGGAGAGGGAAGAATCT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | CD34; Huh7; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000345221.7; ENST00000571307.1; ENST00000617618.4; ENST00000571451.6; ENST00000619223.4; ENST00000262467.10; ENST00000544378.6; ENST00000576905.5; ENST00000572143.1; ENST00000613500.4; ENST00000269280.8; ENST00000572272.6 | ||
| External Link | RMBase: m6A_site_344708 | ||
| mod ID: M6ASITE029832 | Click to Show/Hide the Full List | ||
| mod site | chr17:5584450-5584451:- | [13] | |
| Sequence | GCCTGGAGAGGTCTGAAGAAACCTGGGAGCCAGCAGCCCGG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000571451.6; ENST00000544378.6; ENST00000576905.5; ENST00000619223.4; ENST00000572143.1; ENST00000345221.7; ENST00000262467.10; ENST00000613500.4; ENST00000572272.6; ENST00000571307.1; ENST00000617618.4 | ||
| External Link | RMBase: m6A_site_344709 | ||
Pseudouridine (Pseudo)
| In total 1 m6A sequence/site(s) in this target gene | |||
| mod ID: PSESITE000080 | Click to Show/Hide the Full List | ||
| mod site | chr17:5499572-5499573:- | [16] | |
| Sequence | GGAACCACCTTGGCAAGATATTTACCCACCGGCCATCTCTG | ||
| Transcript ID List | ENST00000544378.6; ENST00000568641.1; ENST00000574406.1 | ||
| External Link | RMBase: Pseudo_site_1894 | ||
References