m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00706)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
STEAP2
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Methyltransferase-like 3 (METTL3) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL3 | ||
| Cell Line | ARPE-19 cell line | Homo sapiens |
|
Treatment: shMETTL3 ARPE-19 cells
Control: shControl ARPE-19 cells
|
GSE202017 | |
| Regulation |
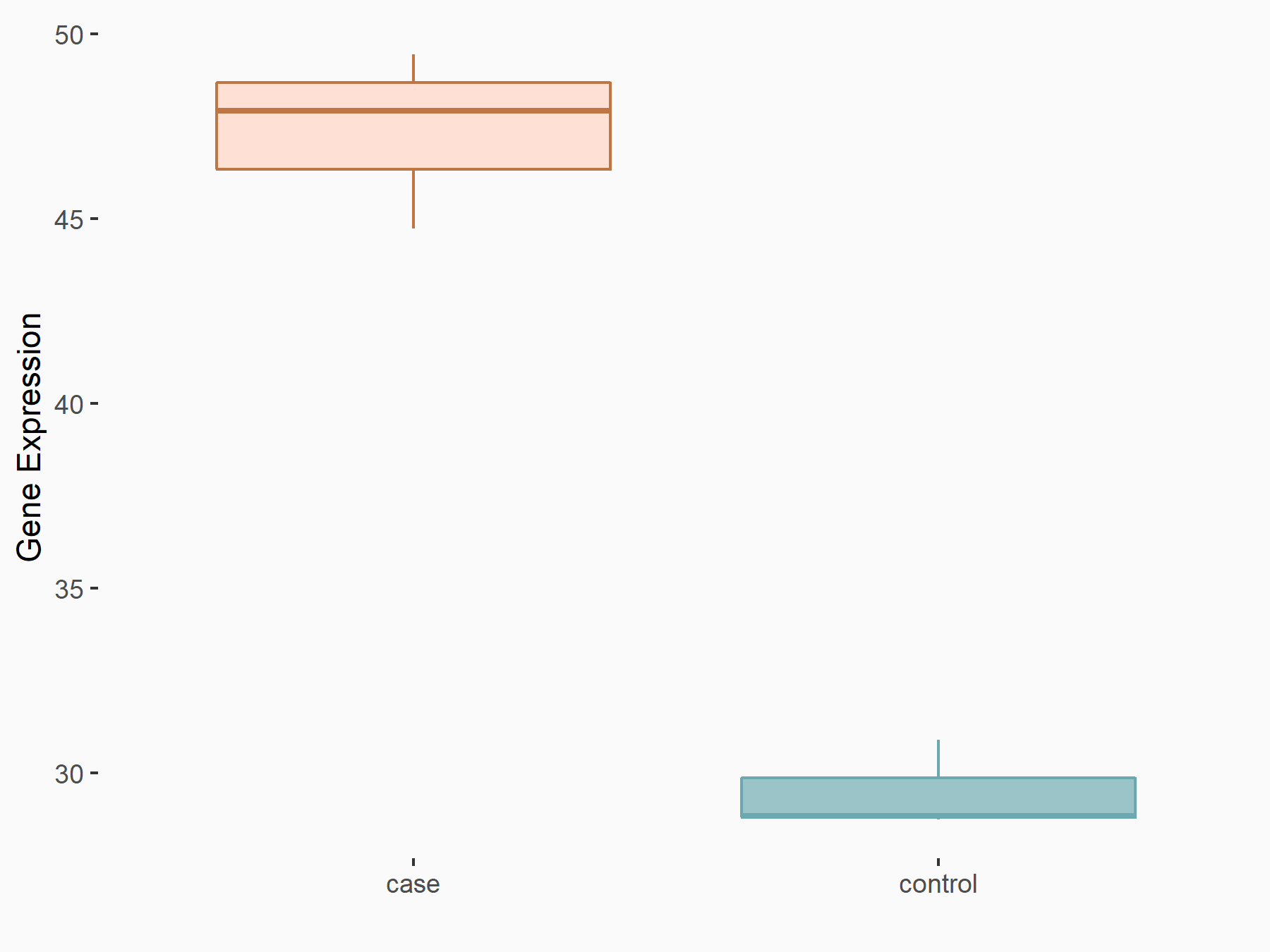 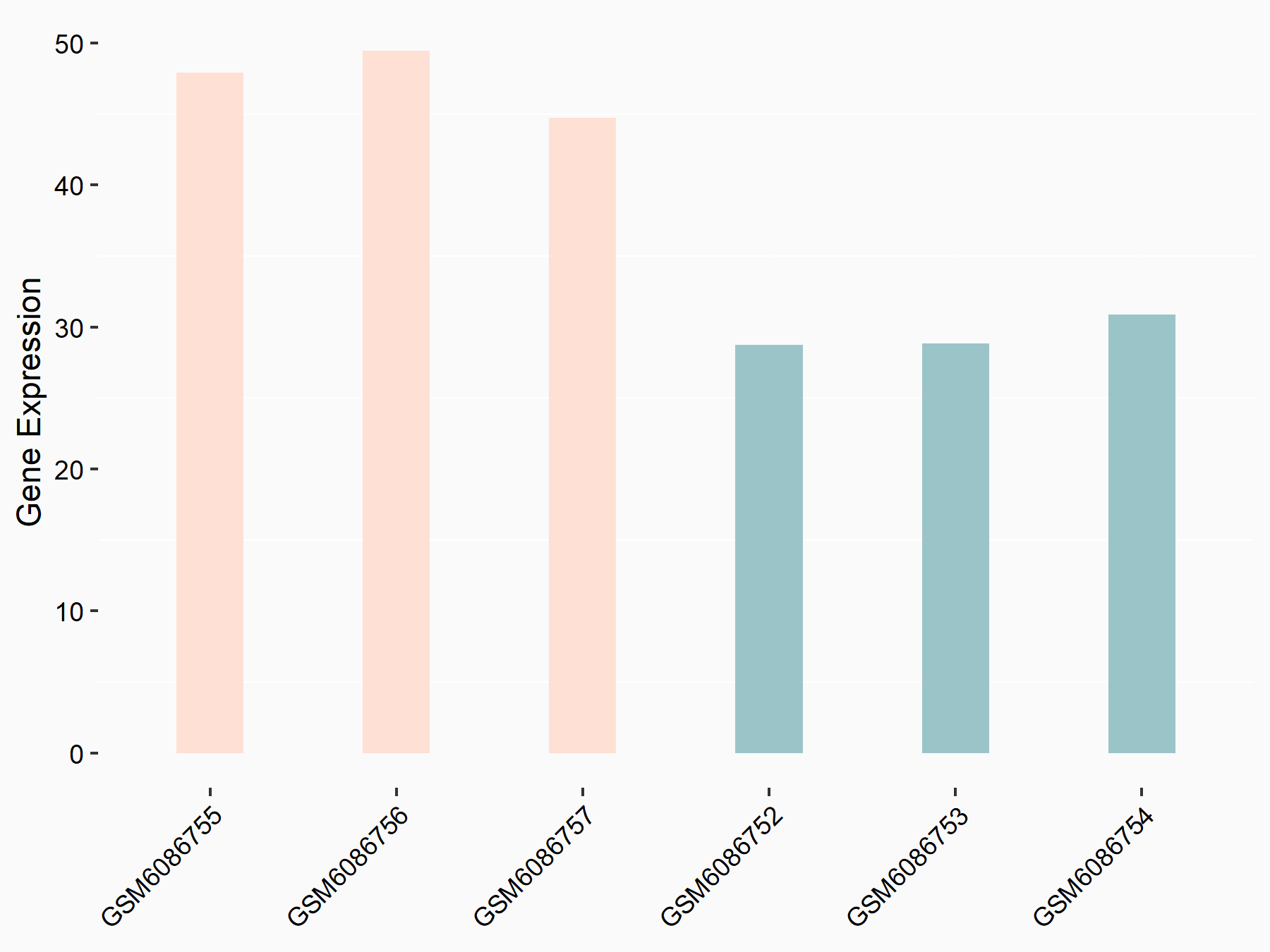 |
logFC: 6.65E-01 p-value: 3.16E-05 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | Metalloreductase STEAP2 (STEAP2) overexpression inhibited papillary thyroid cancer cell proliferation, migration, and invasion in vitro and inhibited lung metastasis and tumorigenicity in vivo. METTL3 stabilized STEAP2 mRNA and regulated STEAP2 expression positively in an m6A-dependent manner. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Papillary thyroid cancer | ICD-11: 2D10.1 | ||
| Pathway Response | Hedgehog signaling pathway | hsa04340 | ||
| Cell Process | Epithelial-to-mesenchymal transition | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
| In-vitro Model | TPC-1 | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_6298 |
| KTC-1 | Thyroid carcinoma | Homo sapiens | CVCL_6300 | |
| B-CPAP | Thyroid gland carcinoma | Homo sapiens | CVCL_0153 | |
| In-vivo Model | BCPAP cells (5×106) were introduced into the mice by means of subcutaneous injection through the flank area. STEAP2-saRNA or NC-saRNA (n = 6 for each group) was given by intratumoral multipoint injection at an interval of 3 days (5 injections in total) using an in vivo transfection reagent (Entranster -in vivo, Engreen, China) as per the vendor-provided protocol. Tumor volume (V) was monitored and calculated as follows: V = (L×W2)/2. For the in vivo tumor metastasis assay, BCPAP cells (5×106 cells) were administrated into mice through the tail vein. STEAP2-saRNA or NC-saRNA (n = 6 for each group) was given via tail vein injection at an interval of 3 days (8 injections in total). | |||
Thyroid Cancer [ICD-11: 2D10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | Metalloreductase STEAP2 (STEAP2) overexpression inhibited papillary thyroid cancer cell proliferation, migration, and invasion in vitro and inhibited lung metastasis and tumorigenicity in vivo. METTL3 stabilized STEAP2 mRNA and regulated STEAP2 expression positively in an m6A-dependent manner. | |||
| Responsed Disease | Papillary thyroid cancer [ICD-11: 2D10.1] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Hedgehog signaling pathway | hsa04340 | ||
| Cell Process | Epithelial-to-mesenchymal transition | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
| In-vitro Model | TPC-1 | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_6298 |
| KTC-1 | Thyroid carcinoma | Homo sapiens | CVCL_6300 | |
| B-CPAP | Thyroid gland carcinoma | Homo sapiens | CVCL_0153 | |
| In-vivo Model | BCPAP cells (5×106) were introduced into the mice by means of subcutaneous injection through the flank area. STEAP2-saRNA or NC-saRNA (n = 6 for each group) was given by intratumoral multipoint injection at an interval of 3 days (5 injections in total) using an in vivo transfection reagent (Entranster -in vivo, Engreen, China) as per the vendor-provided protocol. Tumor volume (V) was monitored and calculated as follows: V = (L×W2)/2. For the in vivo tumor metastasis assay, BCPAP cells (5×106 cells) were administrated into mice through the tail vein. STEAP2-saRNA or NC-saRNA (n = 6 for each group) was given via tail vein injection at an interval of 3 days (8 injections in total). | |||
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00706)
| In total 26 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE080725 | Click to Show/Hide the Full List | ||
| mod site | chr7:90212324-90212325:+ | [2] | |
| Sequence | AGGAGAAAGAAGGCAAGGAGACATTGTCCCAGGTAGGATGT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000402625.6; ENST00000426158.1; ENST00000394626.5; ENST00000287908.7; ENST00000428074.5; ENST00000394632.5; ENST00000394621.7; ENST00000394622.6 | ||
| External Link | RMBase: m6A_site_763965 | ||
| mod ID: M6ASITE080726 | Click to Show/Hide the Full List | ||
| mod site | chr7:90216145-90216146:+ | [3] | |
| Sequence | CTTCAAAAGAAAAGGTATAGACACTGTGGACATCACCTGGG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | MSC | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000394621.7; ENST00000394629.2; ENST00000394626.5; ENST00000482369.1; ENST00000428074.5; ENST00000402625.6; ENST00000287908.7; ENST00000394622.6; ENST00000426158.1; ENST00000394632.5 | ||
| External Link | RMBase: m6A_site_763966 | ||
| mod ID: M6ASITE080727 | Click to Show/Hide the Full List | ||
| mod site | chr7:90216154-90216155:+ | [3] | |
| Sequence | AAAAGGTATAGACACTGTGGACATCACCTGGGAGCTTGTGA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | MSC | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000482369.1; ENST00000394626.5; ENST00000394632.5; ENST00000394629.2; ENST00000428074.5; ENST00000394622.6; ENST00000402625.6; ENST00000287908.7; ENST00000394621.7; ENST00000426158.1 | ||
| External Link | RMBase: m6A_site_763967 | ||
| mod ID: M6ASITE080728 | Click to Show/Hide the Full List | ||
| mod site | chr7:90216205-90216206:+ | [3] | |
| Sequence | AATGTCTAACCTCCCTCTAGACCTAAAGAATCTGAGTGTGC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | MSC | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000287908.7; ENST00000428074.5; ENST00000402625.6; ENST00000394629.2; ENST00000394622.6; ENST00000394626.5; ENST00000394621.7; rmsk_2300462; ENST00000394632.5; ENST00000482369.1; ENST00000426158.1 | ||
| External Link | RMBase: m6A_site_763968 | ||
| mod ID: M6ASITE080729 | Click to Show/Hide the Full List | ||
| mod site | chr7:90225337-90225338:+ | [4] | |
| Sequence | ATGAAGATGCTCTCACAAAAACAAATATAATATTTGTTGCT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | hESC-HEK293T; hESCs; A549 | ||
| Seq Type List | MAZTER-seq; MeRIP-seq; m6A-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000482369.1; ENST00000394626.5; ENST00000402625.6; ENST00000428074.5; ENST00000426158.1; ENST00000394621.7; ENST00000394629.2; ENST00000394622.6; ENST00000394632.5; ENST00000287908.7 | ||
| External Link | RMBase: m6A_site_763969 | ||
| mod ID: M6ASITE080730 | Click to Show/Hide the Full List | ||
| mod site | chr7:90225369-90225370:+ | [5] | |
| Sequence | TTTGTTGCTATACACAGAGAACATTATACCTCCCTGTGGGA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HEK293T; hESCs; A549 | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000394632.5; ENST00000402625.6; ENST00000428074.5; ENST00000394626.5; ENST00000394622.6; ENST00000394621.7; ENST00000287908.7; ENST00000482369.1; ENST00000394629.2 | ||
| External Link | RMBase: m6A_site_763970 | ||
| mod ID: M6ASITE080731 | Click to Show/Hide the Full List | ||
| mod site | chr7:90225389-90225390:+ | [5] | |
| Sequence | ACATTATACCTCCCTGTGGGACCTGAGACATCTGCTTGTGG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HEK293T; hESCs; A549 | ||
| Seq Type List | MeRIP-seq; m6A-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000287908.7; ENST00000394622.6; ENST00000402625.6; ENST00000394632.5; ENST00000428074.5; ENST00000394629.2; ENST00000482369.1; ENST00000394621.7; ENST00000394626.5 | ||
| External Link | RMBase: m6A_site_763971 | ||
| mod ID: M6ASITE080732 | Click to Show/Hide the Full List | ||
| mod site | chr7:90225396-90225397:+ | [5] | |
| Sequence | ACCTCCCTGTGGGACCTGAGACATCTGCTTGTGGGTAAAAT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HEK293T; hESCs; A549 | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000402625.6; ENST00000428074.5; ENST00000287908.7; ENST00000394629.2; ENST00000394621.7; ENST00000482369.1; ENST00000394626.5; ENST00000394632.5; ENST00000394622.6 | ||
| External Link | RMBase: m6A_site_763972 | ||
| mod ID: M6ASITE080733 | Click to Show/Hide the Full List | ||
| mod site | chr7:90225449-90225450:+ | [5] | |
| Sequence | GAGCAATAACATGAGGATAAACCAGTACCCAGAATCCAATG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HEK293T; hESCs; A549 | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000394626.5; ENST00000394632.5; ENST00000394621.7; ENST00000428074.5; ENST00000402625.6; ENST00000394629.2; ENST00000482369.1; ENST00000394622.6; ENST00000287908.7 | ||
| External Link | RMBase: m6A_site_763973 | ||
| mod ID: M6ASITE080734 | Click to Show/Hide the Full List | ||
| mod site | chr7:90225552-90225553:+ | ||
| Sequence | GCTTGGGCACTTCAGTTAGGACCTAAGGATGCCAGCCGGCA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000394629.2; ENST00000394626.5; ENST00000428074.5; ENST00000402625.6; ENST00000482369.1; ENST00000287908.7; ENST00000394621.7; ENST00000394632.5; ENST00000394622.6 | ||
| External Link | RMBase: m6A_site_763974 | ||
| mod ID: M6ASITE080735 | Click to Show/Hide the Full List | ||
| mod site | chr7:90227017-90227018:+ | ||
| Sequence | GCGCGACAACAGGTTATTGAACTTGCCCGCCAGTTGAATTT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000394621.7; ENST00000394629.2; ENST00000402625.6; ENST00000482369.1; ENST00000394622.6; ENST00000287908.7; ENST00000428074.5; ENST00000394632.5; ENST00000394626.5 | ||
| External Link | RMBase: m6A_site_763975 | ||
| mod ID: M6ASITE080736 | Click to Show/Hide the Full List | ||
| mod site | chr7:90227202-90227203:+ | [5] | |
| Sequence | GATTCATCCATATGCTAGAAACCAACAGAGTGACTTTTACA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HEK293T; A549 | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000394632.5; ENST00000394621.7; ENST00000394629.2; ENST00000394626.5; ENST00000394622.6; ENST00000287908.7; ENST00000402625.6 | ||
| External Link | RMBase: m6A_site_763976 | ||
| mod ID: M6ASITE080737 | Click to Show/Hide the Full List | ||
| mod site | chr7:90227214-90227215:+ | [6] | |
| Sequence | TGCTAGAAACCAACAGAGTGACTTTTACAAAATTCCTATAG | ||
| Motif Score | 3.28175 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000394632.5; ENST00000394626.5; ENST00000394629.2; ENST00000394621.7; ENST00000402625.6; ENST00000394622.6; ENST00000287908.7 | ||
| External Link | RMBase: m6A_site_763977 | ||
| mod ID: M6ASITE080738 | Click to Show/Hide the Full List | ||
| mod site | chr7:90227249-90227250:+ | [5] | |
| Sequence | CTATAGAGATTGTGAATAAAACCTTACCTATAGTTGCCATT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HEK293T; A549; Huh7 | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000402625.6; ENST00000394626.5; ENST00000287908.7; ENST00000394621.7; ENST00000394622.6; ENST00000394629.2; ENST00000394632.5 | ||
| External Link | RMBase: m6A_site_763978 | ||
| mod ID: M6ASITE080739 | Click to Show/Hide the Full List | ||
| mod site | chr7:90230010-90230011:+ | [7] | |
| Sequence | TTCAGTGAGCAATGCTTTAAACTGGAGAGAATTCAGTTTTA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000394629.2; ENST00000394626.5; ENST00000402625.6; ENST00000394632.5; ENST00000287908.7; ENST00000394621.7; ENST00000394622.6 | ||
| External Link | RMBase: m6A_site_763979 | ||
| mod ID: M6ASITE080740 | Click to Show/Hide the Full List | ||
| mod site | chr7:90232442-90232443:+ | [7] | |
| Sequence | CAGATTTTATACACCACCAAACTTTGTTCTTGCTCTTGTTT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000394629.2; ENST00000287908.7; ENST00000402625.6; ENST00000394632.5; ENST00000394621.7; ENST00000394626.5; ENST00000394622.6 | ||
| External Link | RMBase: m6A_site_763980 | ||
| mod ID: M6ASITE080741 | Click to Show/Hide the Full List | ||
| mod site | chr7:90236772-90236773:+ | [5] | |
| Sequence | TTCATCCAAAATTAAGGCAGACTGTTTGGATTCTTCCAGTG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HEK293T; A549 | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000402625.6; ENST00000394632.5; ENST00000394629.2; ENST00000287908.7; ENST00000394626.5; ENST00000394622.6; ENST00000394621.7 | ||
| External Link | RMBase: m6A_site_763981 | ||
| mod ID: M6ASITE080742 | Click to Show/Hide the Full List | ||
| mod site | chr7:90236917-90236918:+ | [5] | |
| Sequence | GCAGCTTTGCAGATACCCAGACTGAGCTGGAACTGGAATTT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HEK293T; hESCs; A549; GM12878; Huh7; HEK293A-TOA; MSC | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000287908.7; ENST00000394622.6; ENST00000394621.7; ENST00000394629.2; ENST00000402625.6; ENST00000394632.5; ENST00000394626.5 | ||
| External Link | RMBase: m6A_site_763982 | ||
| mod ID: M6ASITE080743 | Click to Show/Hide the Full List | ||
| mod site | chr7:90236928-90236929:+ | [5] | |
| Sequence | GATACCCAGACTGAGCTGGAACTGGAATTTGTCTTCCTATT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HEK293T; hESCs; A549; GM12878; Huh7; HEK293A-TOA; MSC | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000287908.7; ENST00000394622.6; ENST00000394629.2; ENST00000402625.6; ENST00000394626.5; ENST00000394632.5; ENST00000394621.7 | ||
| External Link | RMBase: m6A_site_763983 | ||
| mod ID: M6ASITE080744 | Click to Show/Hide the Full List | ||
| mod site | chr7:90237177-90237178:+ | [8] | |
| Sequence | AAGAGTAAGGCAGATTAGAGACCAGAAAGACCTTGACTACT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HepG2; HEK293T; A549; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; Huh7; HEK293A-TOA; iSLK; MSC | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000394621.7; ENST00000287908.7; ENST00000394622.6; ENST00000402625.6; ENST00000394632.5; ENST00000394629.2; ENST00000394626.5 | ||
| External Link | RMBase: m6A_site_763984 | ||
| mod ID: M6ASITE080745 | Click to Show/Hide the Full List | ||
| mod site | chr7:90237186-90237187:+ | [8] | |
| Sequence | GCAGATTAGAGACCAGAAAGACCTTGACTACTTCCCTACTT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HepG2; HEK293T; A549; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; Huh7; HEK293A-TOA; iSLK; MSC | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000287908.7; ENST00000394621.7; ENST00000394629.2; ENST00000394632.5; ENST00000402625.6; ENST00000394626.5; ENST00000394622.6 | ||
| External Link | RMBase: m6A_site_763985 | ||
| mod ID: M6ASITE080746 | Click to Show/Hide the Full List | ||
| mod site | chr7:90237310-90237311:+ | [4] | |
| Sequence | CTTTATCCTGATACCATTTAACACTGTCTGAATTAACTAGA | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000394626.5; ENST00000394632.5; ENST00000394621.7; ENST00000402625.6; ENST00000287908.7; ENST00000394629.2; ENST00000394622.6 | ||
| External Link | RMBase: m6A_site_763986 | ||
| mod ID: M6ASITE080747 | Click to Show/Hide the Full List | ||
| mod site | chr7:90237330-90237331:+ | [9] | |
| Sequence | ACACTGTCTGAATTAACTAGACTGCAATAATTCTTTCTTTT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000402625.6; ENST00000394621.7; ENST00000394626.5; ENST00000394632.5; ENST00000394622.6; ENST00000287908.7; ENST00000394629.2 | ||
| External Link | RMBase: m6A_site_763987 | ||
| mod ID: M6ASITE080748 | Click to Show/Hide the Full List | ||
| mod site | chr7:90237380-90237381:+ | [4] | |
| Sequence | TAAAGGATAATGTGCAATTCACATTAAAATTGATTTTCCAT | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000394629.2; ENST00000394622.6; ENST00000394621.7; ENST00000402625.6; ENST00000287908.7; ENST00000394626.5; ENST00000394632.5 | ||
| External Link | RMBase: m6A_site_763988 | ||
| mod ID: M6ASITE080749 | Click to Show/Hide the Full List | ||
| mod site | chr7:90237508-90237509:+ | [9] | |
| Sequence | TGTAATTGGTAATTACTAAAACTCTGTAATCTCCAAAATAT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000394622.6; ENST00000402625.6; ENST00000394629.2; ENST00000394626.5; ENST00000394621.7; ENST00000394632.5; ENST00000287908.7 | ||
| External Link | RMBase: m6A_site_763989 | ||
| mod ID: M6ASITE080750 | Click to Show/Hide the Full List | ||
| mod site | chr7:90237583-90237584:+ | [9] | |
| Sequence | CTCATAGATCTGCCTTATAAACATTTAAATAAAAAGTACTA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000402625.6; ENST00000394632.5; ENST00000287908.7; ENST00000394626.5; ENST00000394629.2; ENST00000394621.7; ENST00000394622.6 | ||
| External Link | RMBase: m6A_site_763990 | ||
References