m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00702)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
MC4R
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Fat mass and obesity-associated protein (FTO) [ERASER]
| Representative RNA-seq result indicating the expression of this target gene regulated by FTO | ||
| Cell Line | Cerebral cortex | Mus musculus |
|
Treatment: METTL3 (f/f, Emx1-cre) cerebral cortex
Control: Wild type cerebral cortex
|
GSE154992 | |
| Regulation |
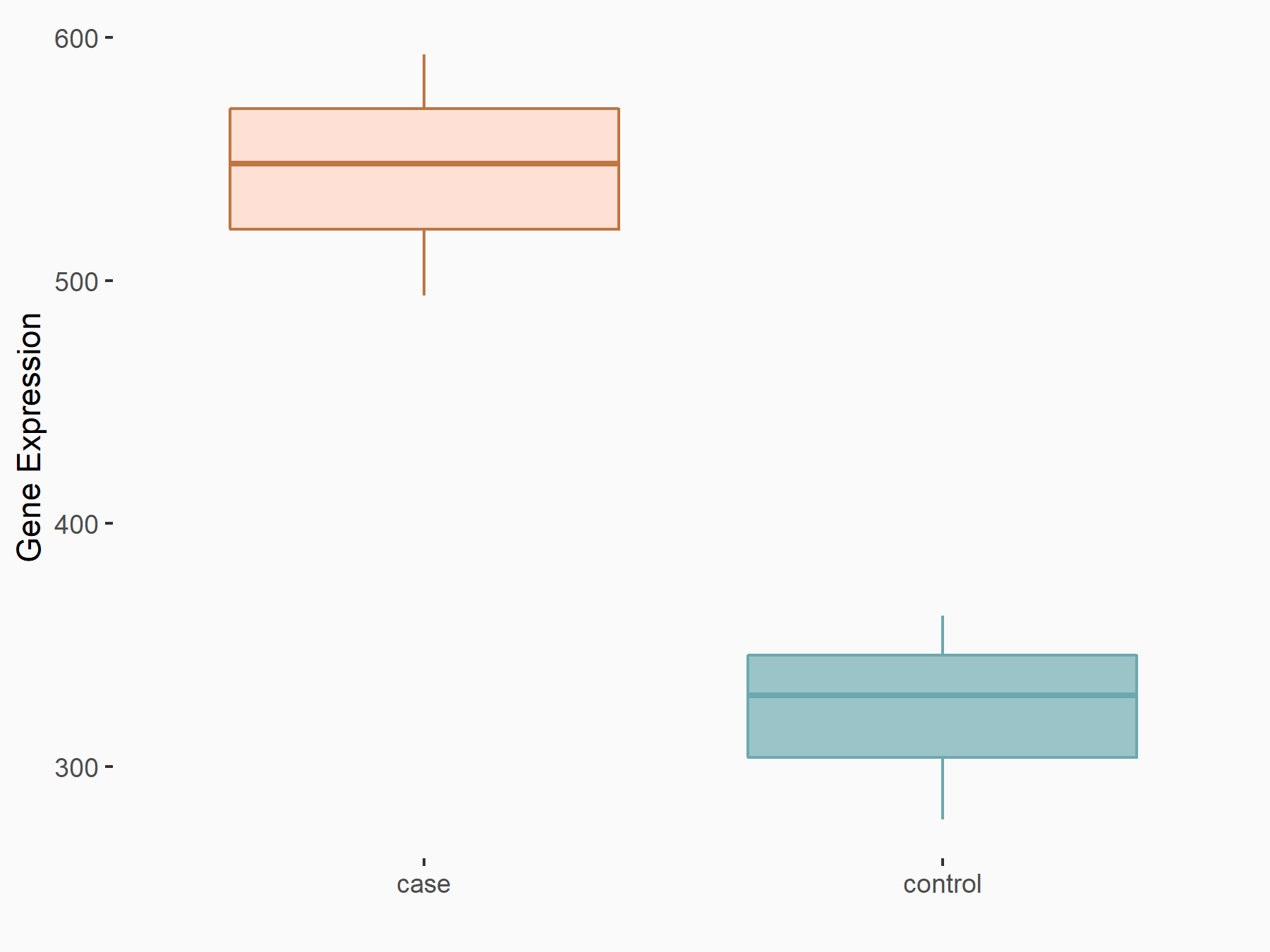 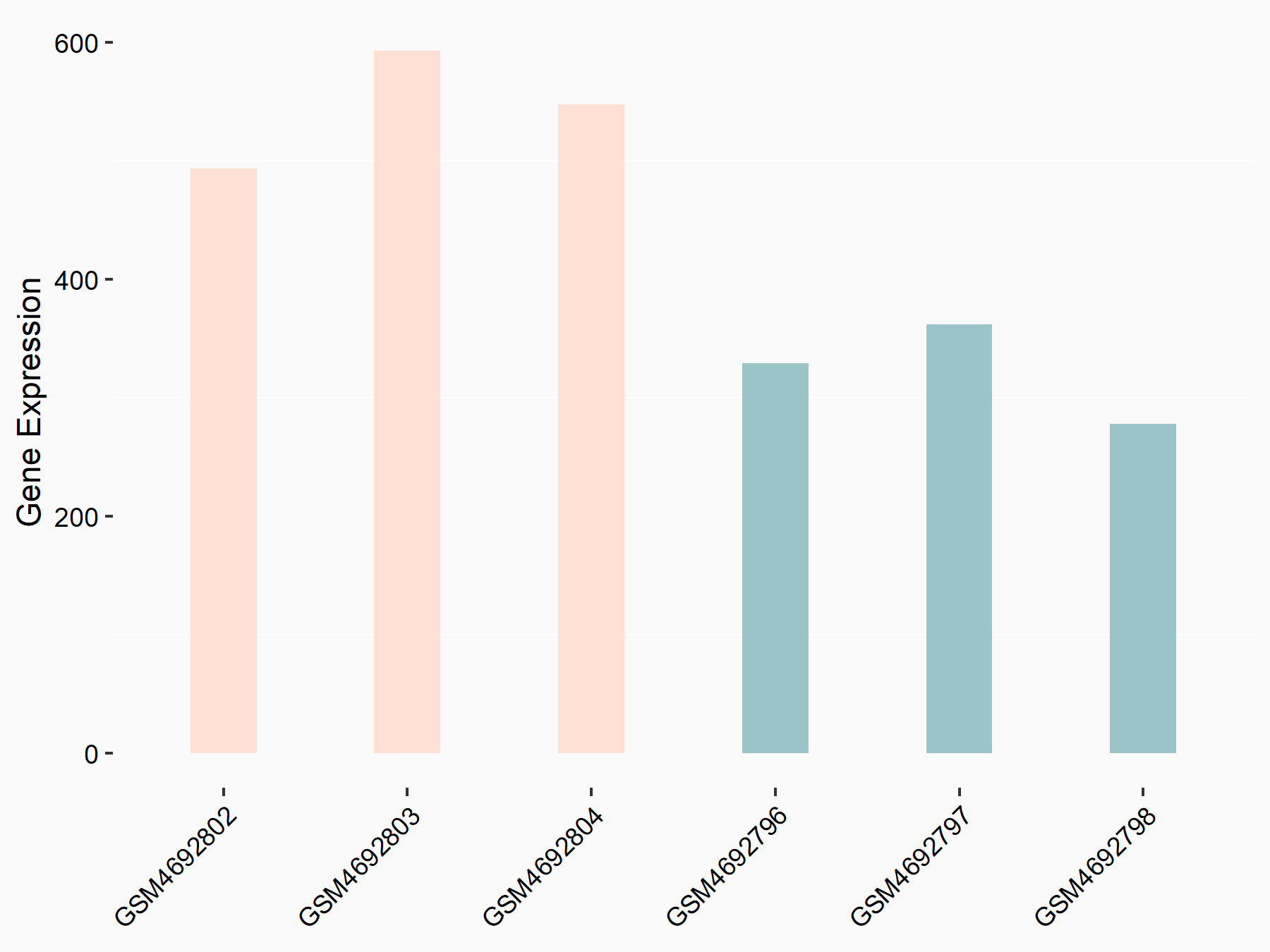 |
logFC: 7.52E-01 p-value: 2.88E-08 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | FTO was downregulated in PCa and its expression level showed a relevance to the prognosis of PCa patients. Additionally, FTO could regulate the proliferation, migration and invasion of PCa via regulating the expression level of Melanocortin receptor 4 (MC4R). | |||
| Target Regulation | Down regulation | |||
| Responsed Disease | Prostate cancer | ICD-11: 2C82 | ||
| Cell Process | Cell proliferation | |||
| Cell migration | ||||
| Cell invasion | ||||
| In-vitro Model | WPMY-1 | Normal | Homo sapiens | CVCL_3814 |
| PC-3 | Prostate carcinoma | Homo sapiens | CVCL_0035 | |
| LNCaP | Prostate carcinoma | Homo sapiens | CVCL_0395 | |
| DU145 | Prostate carcinoma | Homo sapiens | CVCL_0105 | |
| 22Rv1 | Prostate carcinoma | Homo sapiens | CVCL_1045 | |
| In-vivo Model | PCa cells carrying the transfected plasmid were subcutaneously injected into immunodeficient mice at a rate of 1 × 106 cells per mouse according to a previous study. Tumor formation in the two groups of mice was observed and recorded by a designated personnel every day, and the volume of the tumors was measured. The nude mice were sacrificed 21 days after tumor formation, and the tumors were removed to measure their volume and weight. | |||
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | FTO was downregulated in PCa and its expression level showed a relevance to the prognosis of PCa patients. Additionally, FTO could regulate the proliferation, migration and invasion of PCa via regulating the expression level of Melanocortin receptor 4 (MC4R). | |||
| Responsed Disease | Prostate cancer [ICD-11: 2C82] | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Down regulation | |||
| Cell Process | Cell proliferation | |||
| Cell migration | ||||
| Cell invasion | ||||
| In-vitro Model | WPMY-1 | Normal | Homo sapiens | CVCL_3814 |
| PC-3 | Prostate carcinoma | Homo sapiens | CVCL_0035 | |
| LNCaP | Prostate carcinoma | Homo sapiens | CVCL_0395 | |
| DU145 | Prostate carcinoma | Homo sapiens | CVCL_0105 | |
| 22Rv1 | Prostate carcinoma | Homo sapiens | CVCL_1045 | |
| In-vivo Model | PCa cells carrying the transfected plasmid were subcutaneously injected into immunodeficient mice at a rate of 1 × 106 cells per mouse according to a previous study. Tumor formation in the two groups of mice was observed and recorded by a designated personnel every day, and the volume of the tumors was measured. The nude mice were sacrificed 21 days after tumor formation, and the tumors were removed to measure their volume and weight. | |||
References