m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00473)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
HIPK2
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Browse Drug
Methyltransferase-like 3 (METTL3) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL3 | ||
| Cell Line | LX2 cell line | Homo sapiens |
|
Treatment: shMETTL3 LX2 cells
Control: shLuc LX2 cells
|
GSE207909 | |
| Regulation |
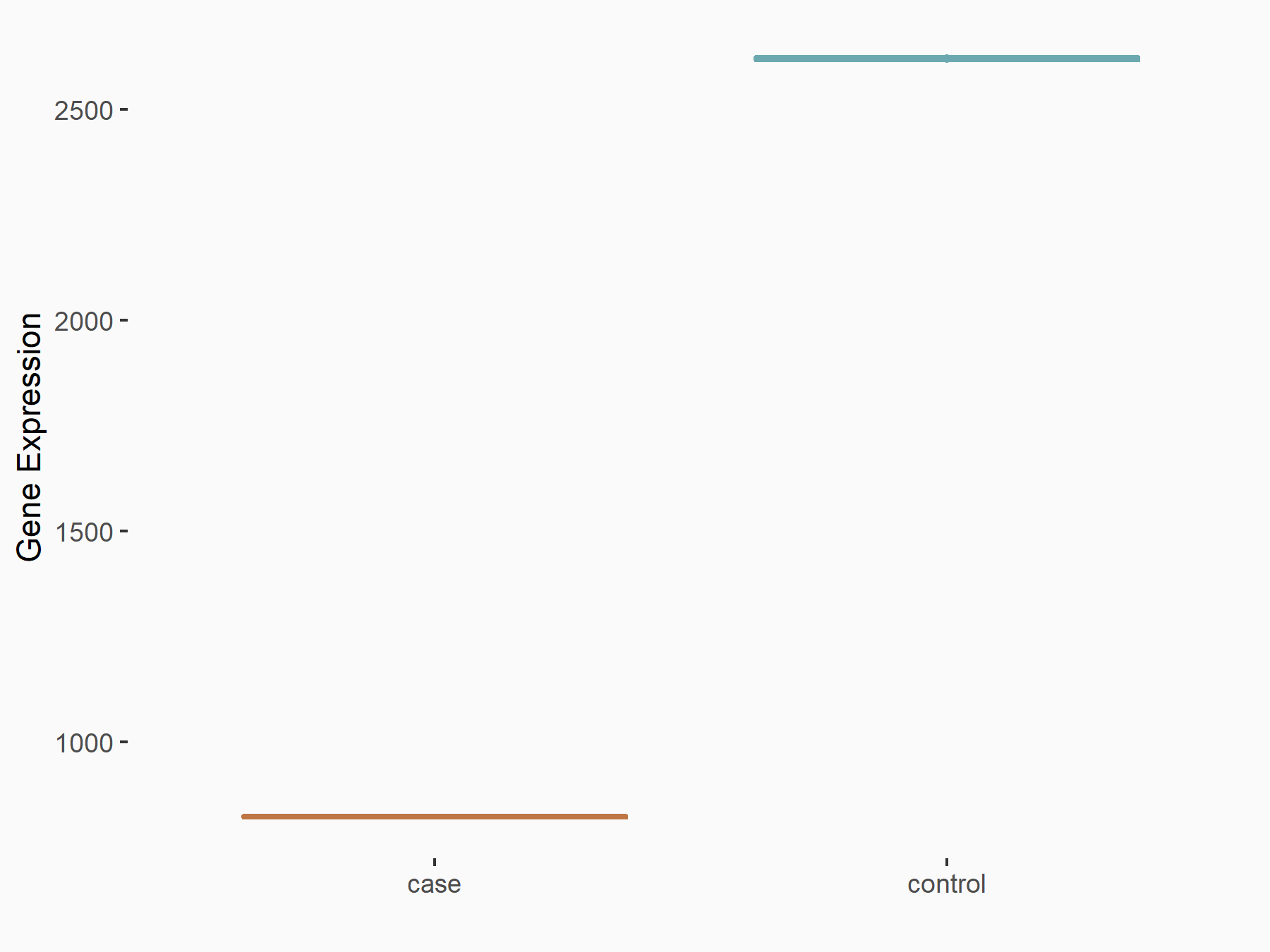  |
logFC: -1.67E+00 p-value: 2.62E-66 |
| More Results | Click to View More RNA-seq Results | |
| Representative RIP-seq result supporting the interaction between HIPK2 and the regulator | ||
| Cell Line | MDA-MB-231 | Homo sapiens |
| Regulation | logFC: 1.33E+00 | GSE60213 |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | METTL3 promotes adriamycin resistance in MCF-7 breast cancer cells by accelerating pri-microRNA-221-3p maturation in a m6A-dependent manner. METTL3 knockdown was shown to reduce the expression of miR-221-3p by reducing pri-miR-221-3p m6A mRNA methylation, reducing the expression of MDR1 and BCRP, and inducing apoptosis. Identified the METTL3/miR-221-3p/Homeodomain-interacting protein kinase 2 (HIPK2)/Che-1 axis as a novel signaling event that will be responsible for resistance of BC cells to ADR. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | ||
| Responsed Drug | Doxil | Approved | ||
| Cell Process | Cell growth and death | |||
| Cell apoptosis | ||||
| In-vitro Model | ADR-resistant MCF-7 (MCF-7/ADR) cells (Human breast cancer doxorubicin-resistant cell line) | |||
| MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 | |
| MCF-10A | Normal | Homo sapiens | CVCL_0598 | |
| In-vivo Model | Cell suspensions (2 × 106 cells/mL) made with MCF-7/ADR cells stably expressing METTL3 and/or miR-221-3p inhibitor were subcutaneously implanted into each mouse. One week later, xenografted mice were injected with 0.1 mL ADR (25 mg/kg, intraperitoneal injection) twice a week. | |||
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | METTL3 promotes adriamycin resistance in MCF-7 breast cancer cells by accelerating pri-microRNA-221-3p maturation in a m6A-dependent manner. METTL3 knockdown was shown to reduce the expression of miR-221-3p by reducing pri-miR-221-3p m6A mRNA methylation, reducing the expression of MDR1 and BCRP, and inducing apoptosis. Identified the METTL3/miR-221-3p/Homeodomain-interacting protein kinase 2 (HIPK2)/Che-1 axis as a novel signaling event that will be responsible for resistance of BC cells to ADR. | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Drug | Doxil | Approved | ||
| Cell Process | Cell growth and death | |||
| Cell apoptosis | ||||
| In-vitro Model | ADR-resistant MCF-7 (MCF-7/ADR) cells (Human breast cancer doxorubicin-resistant cell line) | |||
| MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 | |
| MCF-10A | Normal | Homo sapiens | CVCL_0598 | |
| In-vivo Model | Cell suspensions (2 × 106 cells/mL) made with MCF-7/ADR cells stably expressing METTL3 and/or miR-221-3p inhibitor were subcutaneously implanted into each mouse. One week later, xenografted mice were injected with 0.1 mL ADR (25 mg/kg, intraperitoneal injection) twice a week. | |||
Doxil
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response | [1] | |||
| Response Summary | METTL3 promotes adriamycin resistance in MCF-7 breast cancer cells by accelerating pri-microRNA-221-3p maturation in a m6A-dependent manner. METTL3 knockdown was shown to reduce the expression of miR-221-3p by reducing pri-miR-221-3p m6A mRNA methylation, reducing the expression of MDR1 and BCRP, and inducing apoptosis. Identified the METTL3/miR-221-3p/Homeodomain-interacting protein kinase 2 (HIPK2)/Che-1 axis as a novel signaling event that will be responsible for resistance of BC cells to ADR. | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | ||
| Cell Process | Cell growth and death | |||
| Cell apoptosis | ||||
| In-vitro Model | ADR-resistant MCF-7 (MCF-7/ADR) cells (Human breast cancer doxorubicin-resistant cell line) | |||
| MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 | |
| MCF-10A | Normal | Homo sapiens | CVCL_0598 | |
| In-vivo Model | Cell suspensions (2 × 106 cells/mL) made with MCF-7/ADR cells stably expressing METTL3 and/or miR-221-3p inhibitor were subcutaneously implanted into each mouse. One week later, xenografted mice were injected with 0.1 mL ADR (25 mg/kg, intraperitoneal injection) twice a week. | |||
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00473)
| In total 34 m6A sequence/site(s) in this target gene | |||
| mod ID: A2ISITE002334 | Click to Show/Hide the Full List | ||
| mod site | chr7:139574098-139574099:- | [4] | |
| Sequence | GTGCGGTGGCTCACGCCTGTAGTCCCAGCTTCTCGGGAGCC | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6; rmsk_2390207 | ||
| External Link | RMBase: RNA-editing_site_127674 | ||
| mod ID: A2ISITE002335 | Click to Show/Hide the Full List | ||
| mod site | chr7:139578874-139578875:- | [4] | |
| Sequence | GTTCGAGACCAGCCTGGCCAACATGGCAAAACCCCATCTCT | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6; rmsk_2390222 | ||
| External Link | RMBase: RNA-editing_site_127675 | ||
| mod ID: A2ISITE002336 | Click to Show/Hide the Full List | ||
| mod site | chr7:139634502-139634503:- | [4] | |
| Sequence | AACACTGACACAGGTGGCCTAGGCAGGTGGTGGGCTCGTGG | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: RNA-editing_site_127676 | ||
| mod ID: A2ISITE002337 | Click to Show/Hide the Full List | ||
| mod site | chr7:139640716-139640717:- | [5] | |
| Sequence | GTGGAAGGTGCGGTCAACCAAGATTGTGCCACTGCACTCCA | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8; rmsk_2390332 | ||
| External Link | RMBase: RNA-editing_site_127677 | ||
| mod ID: A2ISITE002338 | Click to Show/Hide the Full List | ||
| mod site | chr7:139640717-139640718:- | [5] | |
| Sequence | GGTGGAAGGTGCGGTCAACCAAGATTGTGCCACTGCACTCC | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; ENST00000428878.6; rmsk_2390332 | ||
| External Link | RMBase: RNA-editing_site_127678 | ||
| mod ID: A2ISITE002339 | Click to Show/Hide the Full List | ||
| mod site | chr7:139640731-139640732:- | [5] | |
| Sequence | GCTTGAGCCCAGGAGGTGGAAGGTGCGGTCAACCAAGATTG | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6; ENST00000342645.7; rmsk_2390332 | ||
| External Link | RMBase: RNA-editing_site_127679 | ||
| mod ID: A2ISITE002340 | Click to Show/Hide the Full List | ||
| mod site | chr7:139640741-139640742:- | [5] | |
| Sequence | AGGGAGGGTTGCTTGAGCCCAGGAGGTGGAAGGTGCGGTCA | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; rmsk_2390332; ENST00000428878.6 | ||
| External Link | RMBase: RNA-editing_site_127680 | ||
| mod ID: A2ISITE002341 | Click to Show/Hide the Full List | ||
| mod site | chr7:139640773-139640774:- | [5] | |
| Sequence | GCCTGTGGTCCCAGCTACTCAGGAGGCTGAGGAGGGAGGGT | ||
| Transcript ID List | rmsk_2390332; ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: RNA-editing_site_127681 | ||
| mod ID: A2ISITE002342 | Click to Show/Hide the Full List | ||
| mod site | chr7:139640830-139640831:- | [5] | |
| Sequence | TTGAGCTCAAGAGCTCAAGAAGAGCCTGGGCAACATGGTGA | ||
| Transcript ID List | ENST00000342645.7; rmsk_2390332; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: RNA-editing_site_127682 | ||
| mod ID: A2ISITE002343 | Click to Show/Hide the Full List | ||
| mod site | chr7:139640831-139640832:- | [5] | |
| Sequence | CTTGAGCTCAAGAGCTCAAGAAGAGCCTGGGCAACATGGTG | ||
| Transcript ID List | ENST00000428878.6; rmsk_2390332; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: RNA-editing_site_127683 | ||
| mod ID: A2ISITE002344 | Click to Show/Hide the Full List | ||
| mod site | chr7:139640834-139640835:- | [5] | |
| Sequence | TTGCTTGAGCTCAAGAGCTCAAGAAGAGCCTGGGCAACATG | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8; rmsk_2390332 | ||
| External Link | RMBase: RNA-editing_site_127684 | ||
| mod ID: A2ISITE002345 | Click to Show/Hide the Full List | ||
| mod site | chr7:139640841-139640842:- | [5] | |
| Sequence | GGAAGGATTGCTTGAGCTCAAGAGCTCAAGAAGAGCCTGGG | ||
| Transcript ID List | ENST00000342645.7; rmsk_2390332; ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: RNA-editing_site_127685 | ||
| mod ID: A2ISITE002346 | Click to Show/Hide the Full List | ||
| mod site | chr7:139640890-139640891:- | [5] | |
| Sequence | GGGTGCAGTGGCTCATACCCATAATCCCAACACTTTGGGAG | ||
| Transcript ID List | ENST00000342645.7; ENST00000406875.8; ENST00000428878.6; rmsk_2390332 | ||
| External Link | RMBase: RNA-editing_site_127686 | ||
| mod ID: A2ISITE002347 | Click to Show/Hide the Full List | ||
| mod site | chr7:139640894-139640895:- | [5] | |
| Sequence | AGCTGGGTGCAGTGGCTCATACCCATAATCCCAACACTTTG | ||
| Transcript ID List | ENST00000342645.7; rmsk_2390332; ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: RNA-editing_site_127687 | ||
| mod ID: A2ISITE002348 | Click to Show/Hide the Full List | ||
| mod site | chr7:139653905-139653906:- | [4] | |
| Sequence | ACCTCGTCTCTACTAAAAATACAAAAATTAGCCGGGCGTGG | ||
| Transcript ID List | ENST00000406875.8; rmsk_2390357; ENST00000428878.6; ENST00000342645.7 | ||
| External Link | RMBase: RNA-editing_site_127688 | ||
| mod ID: A2ISITE002349 | Click to Show/Hide the Full List | ||
| mod site | chr7:139656029-139656030:- | [4] | |
| Sequence | CAACCCTAAGGGGACTTGGAAAGCGAGTTGAGGTCAACAGG | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: RNA-editing_site_127689 | ||
| mod ID: A2ISITE002350 | Click to Show/Hide the Full List | ||
| mod site | chr7:139665965-139665966:- | [5] | |
| Sequence | CTGGGCAGCTCAGTGTTTAAAAAAAAAAAAAAAAAAGGCAG | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: RNA-editing_site_127690 | ||
| mod ID: A2ISITE002351 | Click to Show/Hide the Full List | ||
| mod site | chr7:139666811-139666812:- | [5] | |
| Sequence | CCTCGGCCTCCCAAACTGCTAGGATTATAGGTGTGAGCCAC | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; ENST00000428878.6 | ||
| External Link | RMBase: RNA-editing_site_127691 | ||
| mod ID: A2ISITE002352 | Click to Show/Hide the Full List | ||
| mod site | chr7:139666845-139666846:- | [5] | |
| Sequence | GGTCTAGAACTCCTGACCTCAGGCCGTCTACCTGCCTCGGC | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: RNA-editing_site_127692 | ||
| mod ID: A2ISITE002353 | Click to Show/Hide the Full List | ||
| mod site | chr7:139666870-139666871:- | [5] | |
| Sequence | CGGGTTTCGCCATTTTGGCCAGGCTGGTCTAGAACTCCTGA | ||
| Transcript ID List | ENST00000342645.7; ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: RNA-editing_site_127693 | ||
| mod ID: A2ISITE002354 | Click to Show/Hide the Full List | ||
| mod site | chr7:139666879-139666880:- | [5] | |
| Sequence | CAGCAGAGACGGGTTTCGCCATTTTGGCCAGGCTGGTCTAG | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; ENST00000428878.6 | ||
| External Link | RMBase: RNA-editing_site_127694 | ||
| mod ID: A2ISITE002355 | Click to Show/Hide the Full List | ||
| mod site | chr7:139666938-139666939:- | [5] | |
| Sequence | CCTCCTGAGTAGCTGGGATTACAGGATCCTGCCACTACACC | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: RNA-editing_site_127695 | ||
| mod ID: A2ISITE002356 | Click to Show/Hide the Full List | ||
| mod site | chr7:139666977-139666978:- | [5] | |
| Sequence | CTGCCCCCCAACAAGGTTCAAGCGATTCTTCTGCCTCAGCC | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6; ENST00000342645.7 | ||
| External Link | RMBase: RNA-editing_site_127696 | ||
| mod ID: A2ISITE002357 | Click to Show/Hide the Full List | ||
| mod site | chr7:139666978-139666979:- | [5] | |
| Sequence | TCTGCCCCCCAACAAGGTTCAAGCGATTCTTCTGCCTCAGC | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; ENST00000428878.6 | ||
| External Link | RMBase: RNA-editing_site_127697 | ||
| mod ID: A2ISITE002358 | Click to Show/Hide the Full List | ||
| mod site | chr7:139667017-139667018:- | [5] | |
| Sequence | GGCTGGAGTGCAGTGGCGCAATCTCGGCTCACTGCAACCTC | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: RNA-editing_site_127698 | ||
| mod ID: A2ISITE002359 | Click to Show/Hide the Full List | ||
| mod site | chr7:139670835-139670836:- | [4] | |
| Sequence | GGAAGGCGGAGAGGGCAGTGAGCTGAGATTGTGCCATTGCA | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; rmsk_2390384; ENST00000342645.7 | ||
| External Link | RMBase: RNA-editing_site_127699 | ||
| mod ID: A2ISITE002360 | Click to Show/Hide the Full List | ||
| mod site | chr7:139673983-139673984:- | [5] | |
| Sequence | CTGCCTCCTCAGCTCAAACGATTGTTCTGCCTCAGCCTCCC | ||
| Transcript ID List | ENST00000342645.7; ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: RNA-editing_site_127700 | ||
| mod ID: A2ISITE002361 | Click to Show/Hide the Full List | ||
| mod site | chr7:139673987-139673988:- | [5] | |
| Sequence | ACCTCTGCCTCCTCAGCTCAAACGATTGTTCTGCCTCAGCC | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: RNA-editing_site_127701 | ||
| mod ID: A2ISITE002362 | Click to Show/Hide the Full List | ||
| mod site | chr7:139673988-139673989:- | [5] | |
| Sequence | AACCTCTGCCTCCTCAGCTCAAACGATTGTTCTGCCTCAGC | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: RNA-editing_site_127702 | ||
| mod ID: A2ISITE002363 | Click to Show/Hide the Full List | ||
| mod site | chr7:139674051-139674052:- | [5] | |
| Sequence | TTTTTTTTTGAGACAGTCTCACTCTGTCATCCGGGCTGAAG | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: RNA-editing_site_127703 | ||
| mod ID: A2ISITE002364 | Click to Show/Hide the Full List | ||
| mod site | chr7:139676188-139676189:- | [5] | |
| Sequence | CAGCAGAGATGAGTAGATGCAGCAGAGAGGAGTTGCGGTGT | ||
| Transcript ID List | rmsk_2390392; ENST00000406875.8; ENST00000428878.6; ENST00000342645.7 | ||
| External Link | RMBase: RNA-editing_site_127704 | ||
| mod ID: A2ISITE002365 | Click to Show/Hide the Full List | ||
| mod site | chr7:139728415-139728416:- | [4] | |
| Sequence | CTTCCCTTCCAGCCCTCAGAAGGAACCAGCCCTGCTGACAC | ||
| Transcript ID List | rmsk_2390474; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: RNA-editing_site_127705 | ||
| mod ID: A2ISITE002366 | Click to Show/Hide the Full List | ||
| mod site | chr7:139730661-139730662:- | [4] | |
| Sequence | TTTTAGTTTAAATGAAAATTAGGGCCAGGTGCGATGGCTCA | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: RNA-editing_site_127706 | ||
| mod ID: A2ISITE002367 | Click to Show/Hide the Full List | ||
| mod site | chr7:139730777-139730778:- | [4] | |
| Sequence | TAACTTGATTACCTCTGAAAAGACCCCATTTCCAAAAAAGA | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: RNA-editing_site_127707 | ||
5-methylcytidine (m5C)
N6-methyladenosine (m6A)
| In total 224 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE082531 | Click to Show/Hide the Full List | ||
| mod site | chr7:139561720-139561721:- | [6] | |
| Sequence | GAACTATGTTATAGCTTGTTACTCATAGTTTCTTTTTGATC | ||
| Motif Score | 2.494845238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779627 | ||
| mod ID: M6ASITE082532 | Click to Show/Hide the Full List | ||
| mod site | chr7:139561780-139561781:- | [6] | |
| Sequence | TTTTAATTGTAATATTACTAACTGTAGGGTGAGAAAAAGGG | ||
| Motif Score | 2.590089286 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779628 | ||
| mod ID: M6ASITE082533 | Click to Show/Hide the Full List | ||
| mod site | chr7:139561784-139561785:- | [6] | |
| Sequence | GTTATTTTAATTGTAATATTACTAACTGTAGGGTGAGAAAA | ||
| Motif Score | 2.494845238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779629 | ||
| mod ID: M6ASITE082534 | Click to Show/Hide the Full List | ||
| mod site | chr7:139561865-139561866:- | [6] | |
| Sequence | AATTTATATTAGTCTTGGAAACTAATAATAGCATTGTAAAT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779630 | ||
| mod ID: M6ASITE082535 | Click to Show/Hide the Full List | ||
| mod site | chr7:139561934-139561935:- | [6] | |
| Sequence | TGCTTGTGTTTTTCATCATGACGTCGTGTGCTTCTAAATTA | ||
| Motif Score | 2.833690476 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779631 | ||
| mod ID: M6ASITE082536 | Click to Show/Hide the Full List | ||
| mod site | chr7:139561961-139561962:- | [6] | |
| Sequence | TAACTGATCAAGTCCTTGCTACTAAAATGCTTGTGTTTTTC | ||
| Motif Score | 2.500660714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779632 | ||
| mod ID: M6ASITE082537 | Click to Show/Hide the Full List | ||
| mod site | chr7:139561979-139561980:- | [6] | |
| Sequence | TACAAGAAAACTAATTCTTAACTGATCAAGTCCTTGCTACT | ||
| Motif Score | 2.590089286 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779633 | ||
| mod ID: M6ASITE082538 | Click to Show/Hide the Full List | ||
| mod site | chr7:139561998-139561999:- | [6] | |
| Sequence | ACTTGGCTTTAGAATGTTTTACAAGAAAACTAATTCTTAAC | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | HEK293T; hESC-HEK293T | ||
| Seq Type List | DART-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779634 | ||
| mod ID: M6ASITE082539 | Click to Show/Hide the Full List | ||
| mod site | chr7:139562018-139562019:- | [6] | |
| Sequence | GTTATAAGAATGGATATTTTACTTGGCTTTAGAATGTTTTA | ||
| Motif Score | 2.494845238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779635 | ||
| mod ID: M6ASITE082540 | Click to Show/Hide the Full List | ||
| mod site | chr7:139562094-139562095:- | [6] | |
| Sequence | TTTTGTTTTTTTAAAGACAAACCAATGTGTTGTAGACCTAT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779636 | ||
| mod ID: M6ASITE082541 | Click to Show/Hide the Full List | ||
| mod site | chr7:139562098-139562099:- | [6] | |
| Sequence | CTACTTTTGTTTTTTTAAAGACAAACCAATGTGTTGTAGAC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779637 | ||
| mod ID: M6ASITE082542 | Click to Show/Hide the Full List | ||
| mod site | chr7:139562116-139562117:- | [6] | |
| Sequence | TTTTTTATTTATAAATGTCTACTTTTGTTTTTTTAAAGACA | ||
| Motif Score | 2.500660714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779638 | ||
| mod ID: M6ASITE082543 | Click to Show/Hide the Full List | ||
| mod site | chr7:139562165-139562166:- | [6] | |
| Sequence | GCAACAGTATTTTCTCGTGTACCTCTTTTTCCTATGTGAAT | ||
| Motif Score | 2.8355 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779639 | ||
| mod ID: M6ASITE082544 | Click to Show/Hide the Full List | ||
| mod site | chr7:139562384-139562385:- | [7] | |
| Sequence | ATGAGAACAGGGAAGAGGAGACCCGGCGACACTAAGTCACC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HEK293T; H1A; H1B; hNPCs; hESCs; fibroblasts; A549; Jurkat; GSC-11; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779640 | ||
| mod ID: M6ASITE082545 | Click to Show/Hide the Full List | ||
| mod site | chr7:139562398-139562399:- | [7] | |
| Sequence | ACTCACTCCCCAAAATGAGAACAGGGAAGAGGAGACCCGGC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HEK293T; brain; H1A; H1B; hNPCs; hESCs; fibroblasts; A549; Jurkat; GSC-11; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779641 | ||
| mod ID: M6ASITE082546 | Click to Show/Hide the Full List | ||
| mod site | chr7:139562418-139562419:- | [7] | |
| Sequence | TCAGGACACTGGATAGAAAGACTCACTCCCCAAAATGAGAA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; HEK293T; H1B; H1A; hNPCs; hESCs; fibroblasts; A549; Jurkat | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779642 | ||
| mod ID: M6ASITE082547 | Click to Show/Hide the Full List | ||
| mod site | chr7:139562433-139562434:- | [7] | |
| Sequence | CAACTGCTCATGAATTCAGGACACTGGATAGAAAGACTCAC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HEK293T; hESC-HEK293T; H1B; H1A; hNPCs; hESCs; fibroblasts; A549; Jurkat | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779643 | ||
| mod ID: M6ASITE082548 | Click to Show/Hide the Full List | ||
| mod site | chr7:139562596-139562597:- | [6] | |
| Sequence | ATCAGTGGGTGACGGGGGCAACAAATCAGAGTGACTGGAAG | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | HEK293T; hESC-HEK293T | ||
| Seq Type List | DART-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779644 | ||
| mod ID: M6ASITE082549 | Click to Show/Hide the Full List | ||
| mod site | chr7:139562728-139562729:- | [8] | |
| Sequence | TGTGTCCCAGGCCACTCCGCACACATGGGGCTGAGGCGTGG | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779645 | ||
| mod ID: M6ASITE082550 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563021-139563022:- | [7] | |
| Sequence | ACTTCAAGCTTCTTCCATGGACTTTCCAGGGCACAGTCATC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779646 | ||
| mod ID: M6ASITE082551 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563151-139563152:- | [7] | |
| Sequence | TCCTGTAACTAACAAATGGAACAGAGAGCACACCCCCGCCC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; A549; MM6; Huh7; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779647 | ||
| mod ID: M6ASITE082552 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563160-139563161:- | [9] | |
| Sequence | TTGGTAGCTTCCTGTAACTAACAAATGGAACAGAGAGCACA | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | brain; kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779648 | ||
| mod ID: M6ASITE082553 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563217-139563218:- | [7] | |
| Sequence | GGCTCAGGTTATAAGGAGGAACTTGGGAAGTAGAAAGTGAC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; A549; Huh7; peripheral-blood; HEK293A-TOA; iSLK; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779649 | ||
| mod ID: M6ASITE082554 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563303-139563304:- | [9] | |
| Sequence | CAGCCTTGAGACTGGTGGTCACACCTCCCTGTCAGAGTCGC | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | kidney; hESC-HEK293T | ||
| Seq Type List | m6A-REF-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779650 | ||
| mod ID: M6ASITE082555 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563313-139563314:- | [7] | |
| Sequence | AGGCCCCACTCAGCCTTGAGACTGGTGGTCACACCTCCCTG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; HEK293A-TOA; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779651 | ||
| mod ID: M6ASITE082556 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563362-139563363:- | [7] | |
| Sequence | CCTGGGCCAAGGCACCCAGGACTCCCAGAAAGCGCGAGAGC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; Huh7; HEK293A-TOA; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779652 | ||
| mod ID: M6ASITE082557 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563615-139563616:- | [6] | |
| Sequence | TTTTTCCTTTACATGAATTAACCAAATGAATTTTGTGTCAT | ||
| Motif Score | 2.147452381 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779653 | ||
| mod ID: M6ASITE082558 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563625-139563626:- | [6] | |
| Sequence | GTGTTGCTGTTTTTTCCTTTACATGAATTAACCAAATGAAT | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779654 | ||
| mod ID: M6ASITE082559 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563730-139563731:- | [9] | |
| Sequence | ATTGACAGCGTGTTCTTAGGACAGTCTTTTGGTGGAAATGT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | brain; kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779655 | ||
| mod ID: M6ASITE082560 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563789-139563790:- | [6] | |
| Sequence | CCAGGGAGCTTTTAAAAAATACAAAAAAATACCACATTTGC | ||
| Motif Score | 2.110482143 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779656 | ||
| mod ID: M6ASITE082561 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563827-139563828:- | [7] | |
| Sequence | TAGCAGATAAGACCTTGAAAACTGCAAAACACCTGGGACCA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779657 | ||
| mod ID: M6ASITE082562 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563836-139563837:- | [7] | |
| Sequence | GCATTCCTTTAGCAGATAAGACCTTGAAAACTGCAAAACAC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779658 | ||
| mod ID: M6ASITE082563 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563875-139563876:- | [7] | |
| Sequence | CCACCACCAAACTGCAGAGGACCTGCTGTGACCTAAAGGGC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779659 | ||
| mod ID: M6ASITE082564 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563885-139563886:- | [7] | |
| Sequence | TTCTCTTGTGCCACCACCAAACTGCAGAGGACCTGCTGTGA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779660 | ||
| mod ID: M6ASITE082565 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563954-139563955:- | [7] | |
| Sequence | GGCCCCACTGTCATGCAGAAACAGACTGGGGGAATGGCAGT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779661 | ||
| mod ID: M6ASITE082566 | Click to Show/Hide the Full List | ||
| mod site | chr7:139563983-139563984:- | [7] | |
| Sequence | GAACAAGAAGATGCTTCGAGACAGGAATGGGCCCCACTGTC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779662 | ||
| mod ID: M6ASITE082567 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564001-139564002:- | [7] | |
| Sequence | AAACAGACATCATATTTGGAACAAGAAGATGCTTCGAGACA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779663 | ||
| mod ID: M6ASITE082568 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564019-139564020:- | [7] | |
| Sequence | TCCATTGAGACCCACTCTAAACAGACATCATATTTGGAACA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779664 | ||
| mod ID: M6ASITE082569 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564030-139564031:- | [7] | |
| Sequence | GCTCAGCCTGGTCCATTGAGACCCACTCTAAACAGACATCA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779665 | ||
| mod ID: M6ASITE082570 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564061-139564062:- | [7] | |
| Sequence | CTGAGCAGGAGGAGGCAGAGACAGAGGGGCAGCTCAGCCTG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779666 | ||
| mod ID: M6ASITE082571 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564111-139564112:- | [7] | |
| Sequence | TCTAGTTCGGTGGGAGGGGGACCTTAGCATCCTCTCAGAGA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779667 | ||
| mod ID: M6ASITE082572 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564139-139564140:- | [7] | |
| Sequence | TTACTCATTGTCCAGCTTAGACTCAAAGTCTAGTTCGGTGG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779668 | ||
| mod ID: M6ASITE082573 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564157-139564158:- | [6] | |
| Sequence | AAAGGCTATGTTTACGTTTTACTCATTGTCCAGCTTAGACT | ||
| Motif Score | 2.494845238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779669 | ||
| mod ID: M6ASITE082574 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564164-139564165:- | [6] | |
| Sequence | CAATGTAAAAGGCTATGTTTACGTTTTACTCATTGTCCAGC | ||
| Motif Score | 2.046785714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779670 | ||
| mod ID: M6ASITE082575 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564325-139564326:- | [9] | |
| Sequence | ATTCAGGTGACCATGAAGGCACAGCTGCTACTTCTGGGCCG | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779671 | ||
| mod ID: M6ASITE082576 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564466-139564467:- | [9] | |
| Sequence | TCTTCAAGGCCTTCTCTCTGACATCTTGGAAGAGTCATTGA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | brain | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779672 | ||
| mod ID: M6ASITE082577 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564576-139564577:- | [7] | |
| Sequence | TTCCACGTGAACTCAAGAGAACATGAAAGGCAATCCAGATG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779673 | ||
| mod ID: M6ASITE082578 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564586-139564587:- | [7] | |
| Sequence | GATTATTACTTTCCACGTGAACTCAAGAGAACATGAAAGGC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779674 | ||
| mod ID: M6ASITE082579 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564641-139564642:- | [7] | |
| Sequence | AGGCAAGTGTGTTGTCCCAGACTCTTCTGTAGTCCAGCATG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779675 | ||
| mod ID: M6ASITE082580 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564703-139564704:- | [7] | |
| Sequence | TTTATAACTGACATCCGCAAACCCCAGTGAATGCCTCTTAG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779676 | ||
| mod ID: M6ASITE082581 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564713-139564714:- | [6] | |
| Sequence | AAAAGTTCTGTTTATAACTGACATCCGCAAACCCCAGTGAA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779677 | ||
| mod ID: M6ASITE082582 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564758-139564759:- | [8] | |
| Sequence | GTTAAAGTGTTTTATAGTTTACATTTAGACTGGTACTTTTT | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779678 | ||
| mod ID: M6ASITE082583 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564824-139564825:- | [8] | |
| Sequence | ATTTTAAATGAGTAAACTAAACAATTGACTGTGGATACTTA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779679 | ||
| mod ID: M6ASITE082584 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564887-139564888:- | [6] | |
| Sequence | AAGTATATATGTTTTTAGCTACTGTAAAATGCTGTTAGCCT | ||
| Motif Score | 2.500660714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779680 | ||
| mod ID: M6ASITE082585 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564914-139564915:- | [8] | |
| Sequence | CAAATGCGATGTTCAAATGCACATGTTAAGTATATATGTTT | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779681 | ||
| mod ID: M6ASITE082586 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564937-139564938:- | [6] | |
| Sequence | GATTATTCTTTGTGTGTTGTACTCAAATGCGATGTTCAAAT | ||
| Motif Score | 3.278136905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779682 | ||
| mod ID: M6ASITE082587 | Click to Show/Hide the Full List | ||
| mod site | chr7:139564982-139564983:- | [8] | |
| Sequence | TCTGAAAATAGCTCATCTTTACAACAAAAATTAAACCAAGG | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779683 | ||
| mod ID: M6ASITE082588 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565037-139565038:- | [6] | |
| Sequence | GTGGGCTGAAAATAATGATTACTTCATACCCCCGGTCATCG | ||
| Motif Score | 2.494845238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779684 | ||
| mod ID: M6ASITE082589 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565071-139565072:- | [7] | |
| Sequence | CTTGATCTTGGAATTTAAAGACTATGAATTCTCTGTGGGCT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779685 | ||
| mod ID: M6ASITE082590 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565170-139565171:- | [7] | |
| Sequence | AGATACATTATCTTACACAAACTGTGACCTAATGGCAATAA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779686 | ||
| mod ID: M6ASITE082591 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565176-139565177:- | [6] | |
| Sequence | GGTTTTAGATACATTATCTTACACAAACTGTGACCTAATGG | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779687 | ||
| mod ID: M6ASITE082592 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565225-139565226:- | [8] | |
| Sequence | TATTCTGGAATTCTGGCAGAACACCTAAAGATTTTTTTTTT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779688 | ||
| mod ID: M6ASITE082593 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565538-139565539:- | [9] | |
| Sequence | GGTATGAGATTAACAGAAATACAGATGCATTTTTATTTTGA | ||
| Motif Score | 2.110482143 | ||
| Cell/Tissue List | brain | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779689 | ||
| mod ID: M6ASITE082594 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565546-139565547:- | [9] | |
| Sequence | CTTGCCCTGGTATGAGATTAACAGAAATACAGATGCATTTT | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | brain | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779690 | ||
| mod ID: M6ASITE082595 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565602-139565603:- | [7] | |
| Sequence | ACAATTGTATAAACAGGAAAACCAGCCAGCTTTCATGATAA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779691 | ||
| mod ID: M6ASITE082596 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565610-139565611:- | [7] | |
| Sequence | AAATAGCAACAATTGTATAAACAGGAAAACCAGCCAGCTTT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779692 | ||
| mod ID: M6ASITE082597 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565668-139565669:- | [10] | |
| Sequence | GAAAGACAAGACTTAGAGGAACAAAAGAATGTTTTCTTTTG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | hNPCs | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779693 | ||
| mod ID: M6ASITE082598 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565678-139565679:- | [10] | |
| Sequence | AGAGGTGGAAGAAAGACAAGACTTAGAGGAACAAAAGAATG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | hNPCs | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779694 | ||
| mod ID: M6ASITE082599 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565683-139565684:- | [10] | |
| Sequence | GAAGTAGAGGTGGAAGAAAGACAAGACTTAGAGGAACAAAA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | hNPCs | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779695 | ||
| mod ID: M6ASITE082600 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565751-139565752:- | [11] | |
| Sequence | TGTGGGTCTGGTGTCCAGGAACTTTTTTCTTTCTGTTAAAA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779696 | ||
| mod ID: M6ASITE082601 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565810-139565811:- | [11] | |
| Sequence | ACTCTTCAGCTCTGGGCAGAACCAGAGGCAGGGTTCACACC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779697 | ||
| mod ID: M6ASITE082602 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565856-139565857:- | [9] | |
| Sequence | TGCACTTGATAATTATCCTGACAGCTCTGATCTCTGTAATA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | liver | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779698 | ||
| mod ID: M6ASITE082603 | Click to Show/Hide the Full List | ||
| mod site | chr7:139565878-139565879:- | [6] | |
| Sequence | TTTTCAAAATGCTATTTTTTACTGCACTTGATAATTATCCT | ||
| Motif Score | 2.494845238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779699 | ||
| mod ID: M6ASITE082604 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566008-139566009:- | [6] | |
| Sequence | CTAGCTTTAGTAGTGCTGCTACAACCAGCTCTTATAAGTAA | ||
| Motif Score | 2.078666667 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779700 | ||
| mod ID: M6ASITE082605 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566033-139566034:- | [6] | |
| Sequence | TTTAGATTTCTTTCCAGCATACCAACTAGCTTTAGTAGTGC | ||
| Motif Score | 2.089839286 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779701 | ||
| mod ID: M6ASITE082606 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566160-139566161:- | [7] | |
| Sequence | CCGTGGGACTCAGCTAAAGGACTGTGCAAAGAGGGGGCTCC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779702 | ||
| mod ID: M6ASITE082607 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566173-139566174:- | [7] | |
| Sequence | GCCATCCCATGCACCGTGGGACTCAGCTAAAGGACTGTGCA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779703 | ||
| mod ID: M6ASITE082608 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566210-139566211:- | [6] | |
| Sequence | CTGAGCAAAGAAAGGCCTTTACACAGCATCACCCTGTGCCA | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779704 | ||
| mod ID: M6ASITE082609 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566249-139566250:- | [9] | |
| Sequence | TGAGCTTGCAGCCATTAGCCACAGCTGTCTCCTGCATGTCT | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779705 | ||
| mod ID: M6ASITE082610 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566271-139566272:- | [7] | |
| Sequence | TGAGTCTGCCCAGCTCCAGAACTGAGCTTGCAGCCATTAGC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779706 | ||
| mod ID: M6ASITE082611 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566294-139566295:- | [7] | |
| Sequence | GAGGGGCGAGGAGGGCTTGAACCTGAGTCTGCCCAGCTCCA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779707 | ||
| mod ID: M6ASITE082612 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566338-139566339:- | [7] | |
| Sequence | GTCCCCCTACAGCCAAGGAAACTGCCCAGAGCCACACAACT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779708 | ||
| mod ID: M6ASITE082613 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566466-139566467:- | [7] | |
| Sequence | GTGGCCTCATATTTCTATGAACCATTTAGTGATACTCCCGC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779709 | ||
| mod ID: M6ASITE082614 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566731-139566732:- | [6] | |
| Sequence | ATTTGTTGAATGCTTTCATGACTCCCGGGCTAAGGCCTTTA | ||
| Motif Score | 3.28175 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779710 | ||
| mod ID: M6ASITE082615 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566816-139566817:- | [7] | |
| Sequence | ATCTCAGATCCCTGTGTTGAACATGACACTGACTGTCCCTT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779711 | ||
| mod ID: M6ASITE082616 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566863-139566864:- | [7] | |
| Sequence | TTCCCGATGCCCCCCGCTGGACTTGCCCAAGCCTCTGGGAG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779712 | ||
| mod ID: M6ASITE082617 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566904-139566905:- | [7] | |
| Sequence | TGCAGGGCTCCCGTCCCAGAACACCAGCACCAGAGAGGGTC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779713 | ||
| mod ID: M6ASITE082618 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566955-139566956:- | [7] | |
| Sequence | CCAGGCCGTTCTTCCCTCAAACCCAGTGAGAGTTTGCAGAG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779714 | ||
| mod ID: M6ASITE082619 | Click to Show/Hide the Full List | ||
| mod site | chr7:139566978-139566979:- | [7] | |
| Sequence | TCTGAAAAGTTCAAAAAAGAACTCCAGGCCGTTCTTCCCTC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779715 | ||
| mod ID: M6ASITE082620 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567032-139567033:- | [7] | |
| Sequence | CACCGTGGCTCACTAGAGGGACCCAGCATAGTAGAGGTTTC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779716 | ||
| mod ID: M6ASITE082621 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567071-139567072:- | [7] | |
| Sequence | AGGATTTAAATGCAAAAAAAACCTATTGGAGGCTTTTGGCA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779717 | ||
| mod ID: M6ASITE082622 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567093-139567094:- | [7] | |
| Sequence | AGAGAATGGAAGGCGGTGAAACAGGATTTAAATGCAAAAAA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779718 | ||
| mod ID: M6ASITE082623 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567136-139567137:- | [7] | |
| Sequence | TTCTCATCTCCACAGACCAGACTCAGTAAAATCTCAGGCCA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779719 | ||
| mod ID: M6ASITE082624 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567141-139567142:- | [7] | |
| Sequence | CTCACTTCTCATCTCCACAGACCAGACTCAGTAAAATCTCA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779720 | ||
| mod ID: M6ASITE082625 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567164-139567165:- | [7] | |
| Sequence | TTCCACTTTGATGCCTTAGAACTCTCACTTCTCATCTCCAC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779721 | ||
| mod ID: M6ASITE082626 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567318-139567319:- | [11] | |
| Sequence | GATGCTGCTGCTATCGGGAGACAAGGTGCCATACCAATACG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779722 | ||
| mod ID: M6ASITE082627 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567378-139567379:- | [11] | |
| Sequence | AAAAATGCATTTATTTCAAAACTGTGCTATTCTTTTAAGAG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779723 | ||
| mod ID: M6ASITE082628 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567502-139567503:- | [7] | |
| Sequence | AAAGACGCCTCCAGAAATGGACAAAATGGCCTTCCCTTCGT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779724 | ||
| mod ID: M6ASITE082629 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567531-139567532:- | [7] | |
| Sequence | TTCCTTTCTTTAGCCAAAGAACCCTTCTCAAAGACGCCTCC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779725 | ||
| mod ID: M6ASITE082630 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567555-139567556:- | [7] | |
| Sequence | AAATTGGAAAGGAAGGTGAAACTGTTCCTTTCTTTAGCCAA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779726 | ||
| mod ID: M6ASITE082631 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567603-139567604:- | [7] | |
| Sequence | TTCTGATGGGGAAGCTTCAAACTTGAGCAGGCCAGAGATGT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779727 | ||
| mod ID: M6ASITE082632 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567629-139567630:- | [9] | |
| Sequence | TTTTCCTTCTGGTGTGTTTTACAGACTTCTGATGGGGAAGC | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779728 | ||
| mod ID: M6ASITE082633 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567824-139567825:- | [7] | |
| Sequence | CCTGTCAAGTAGACCCTAGGACAGAAAATGGAAAGAAGGAA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779729 | ||
| mod ID: M6ASITE082634 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567832-139567833:- | [7] | |
| Sequence | CTTGTTGGCCTGTCAAGTAGACCCTAGGACAGAAAATGGAA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779730 | ||
| mod ID: M6ASITE082635 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567861-139567862:- | [7] | |
| Sequence | AGGGCAGGGTGGGGTGTGGGACCACCACTCTTGTTGGCCTG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; peripheral-blood; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779731 | ||
| mod ID: M6ASITE082636 | Click to Show/Hide the Full List | ||
| mod site | chr7:139567929-139567930:- | [7] | |
| Sequence | GTTGAGAGAGCGACGTGGGGACCCAGCTCGCCCCAGCTTTT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779732 | ||
| mod ID: M6ASITE082637 | Click to Show/Hide the Full List | ||
| mod site | chr7:139568000-139568001:- | [7] | |
| Sequence | TGGAACAAACTTTCTTGGAAACTGGAGGGAGGAGATGAGGA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; Huh7; peripheral-blood | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779733 | ||
| mod ID: M6ASITE082638 | Click to Show/Hide the Full List | ||
| mod site | chr7:139568016-139568017:- | [7] | |
| Sequence | AGCATGTGTGAGTGAGTGGAACAAACTTTCTTGGAAACTGG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T; Huh7; peripheral-blood | ||
| Seq Type List | m6A-seq; MAZTER-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779734 | ||
| mod ID: M6ASITE082639 | Click to Show/Hide the Full List | ||
| mod site | chr7:139568068-139568069:- | [7] | |
| Sequence | TGGCAAACACTCATATTGGAACAAGCTTGGGGTGGAAGATT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; hESC-HEK293T; Huh7; peripheral-blood | ||
| Seq Type List | m6A-seq; MAZTER-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779735 | ||
| mod ID: M6ASITE082640 | Click to Show/Hide the Full List | ||
| mod site | chr7:139568082-139568083:- | [7] | |
| Sequence | TTGGACCTTGGGAATGGCAAACACTCATATTGGAACAAGCT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; Huh7; peripheral-blood; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779736 | ||
| mod ID: M6ASITE082641 | Click to Show/Hide the Full List | ||
| mod site | chr7:139568098-139568099:- | [7] | |
| Sequence | GAACGATTTCCCTTGGTTGGACCTTGGGAATGGCAAACACT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; Huh7; peripheral-blood; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779737 | ||
| mod ID: M6ASITE082642 | Click to Show/Hide the Full List | ||
| mod site | chr7:139568158-139568159:- | [7] | |
| Sequence | CCAACGTGTGAGACGGATGGACATCAGGAAGGGAAGGGGAG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; peripheral-blood; GSC-11; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779738 | ||
| mod ID: M6ASITE082643 | Click to Show/Hide the Full List | ||
| mod site | chr7:139568181-139568182:- | [7] | |
| Sequence | AGAAGACTCAGCCCGCCCAGACACCAACGTGTGAGACGGAT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; H1B; peripheral-blood; GSC-11; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779739 | ||
| mod ID: M6ASITE082644 | Click to Show/Hide the Full List | ||
| mod site | chr7:139568196-139568197:- | [7] | |
| Sequence | TGTCCTCTTCAACCTAGAAGACTCAGCCCGCCCAGACACCA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; peripheral-blood; GSC-11; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779740 | ||
| mod ID: M6ASITE082645 | Click to Show/Hide the Full List | ||
| mod site | chr7:139568282-139568283:- | [7] | |
| Sequence | AAGCGGCATTCATCTCCTGGACTGTTAGCCTTTCTAGTCTT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779741 | ||
| mod ID: M6ASITE082646 | Click to Show/Hide the Full List | ||
| mod site | chr7:139568885-139568886:- | [7] | |
| Sequence | GCCCCACAGACGCTCAGCAAACATTAGTGCACATTCTCCTA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779742 | ||
| mod ID: M6ASITE082647 | Click to Show/Hide the Full List | ||
| mod site | chr7:139568994-139568995:- | [7] | |
| Sequence | TGCTCACACGCTGTCCCCAGACCCACAAAGTGCTAGGCCCC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779743 | ||
| mod ID: M6ASITE082648 | Click to Show/Hide the Full List | ||
| mod site | chr7:139569047-139569048:- | [9] | |
| Sequence | ATCCCCACTGGGCTCCGGCCACATCTTCAGTGCACTGTGCT | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | liver | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779744 | ||
| mod ID: M6ASITE082649 | Click to Show/Hide the Full List | ||
| mod site | chr7:139569118-139569119:- | [7] | |
| Sequence | GGAGGCCAGAGAAACCCTGAACAAAGCTGGGCGGCTGCTCA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779745 | ||
| mod ID: M6ASITE082650 | Click to Show/Hide the Full List | ||
| mod site | chr7:139569125-139569126:- | [7] | |
| Sequence | GACAGCAGGAGGCCAGAGAAACCCTGAACAAAGCTGGGCGG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779746 | ||
| mod ID: M6ASITE082651 | Click to Show/Hide the Full List | ||
| mod site | chr7:139569144-139569145:- | [7] | |
| Sequence | AGCTGGAAGTGGGTGGAGGGACAGCAGGAGGCCAGAGAAAC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779747 | ||
| mod ID: M6ASITE082652 | Click to Show/Hide the Full List | ||
| mod site | chr7:139569378-139569379:- | [8] | |
| Sequence | GTGACCCCATCCGCTTCCCCACAATCCATCCTTTTGCCATC | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779748 | ||
| mod ID: M6ASITE082653 | Click to Show/Hide the Full List | ||
| mod site | chr7:139569489-139569490:- | [8] | |
| Sequence | CCTTAGCCAGCTTCTCTCCCACATCCTCAGAGCTCTCCTGT | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779749 | ||
| mod ID: M6ASITE082654 | Click to Show/Hide the Full List | ||
| mod site | chr7:139569999-139570000:- | [6] | |
| Sequence | GCTACTTACTCTTTCACCTGACATTTTCTTTCCTTTTATTC | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779750 | ||
| mod ID: M6ASITE082655 | Click to Show/Hide the Full List | ||
| mod site | chr7:139570077-139570078:- | [7] | |
| Sequence | CTGATCTTTTAGGGGGAAAGACAGCTTAAAATGTTCTTTTC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779751 | ||
| mod ID: M6ASITE082656 | Click to Show/Hide the Full List | ||
| mod site | chr7:139570124-139570125:- | [9] | |
| Sequence | GCACCCCCAGATTCCTGGGCACAGTTCATTTCCAGCCCTTT | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779752 | ||
| mod ID: M6ASITE082657 | Click to Show/Hide the Full List | ||
| mod site | chr7:139570361-139570362:- | [12] | |
| Sequence | GGGAAGCGCAGCCCTCTGAGACAGCAGGACAATGGTCAGTT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779753 | ||
| mod ID: M6ASITE082658 | Click to Show/Hide the Full List | ||
| mod site | chr7:139570518-139570519:- | [9] | |
| Sequence | GGCCTCAGCGAGAAGGAAGGACACCATGACTGCTCCATGCT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | brain | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779754 | ||
| mod ID: M6ASITE082659 | Click to Show/Hide the Full List | ||
| mod site | chr7:139570551-139570552:- | [13] | |
| Sequence | AACAGGTCCCCAAACTGGAAACAGCTTAGTCCAGGCCTCAG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | MT4 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779755 | ||
| mod ID: M6ASITE082660 | Click to Show/Hide the Full List | ||
| mod site | chr7:139570558-139570559:- | [13] | |
| Sequence | ATAAAGAAACAGGTCCCCAAACTGGAAACAGCTTAGTCCAG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | MT4 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779756 | ||
| mod ID: M6ASITE082661 | Click to Show/Hide the Full List | ||
| mod site | chr7:139570570-139570571:- | [13] | |
| Sequence | AAATTCTTACTTATAAAGAAACAGGTCCCCAAACTGGAAAC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | MT4 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779757 | ||
| mod ID: M6ASITE082662 | Click to Show/Hide the Full List | ||
| mod site | chr7:139570609-139570610:- | [13] | |
| Sequence | GCTGTGAAATAACTCTGGAAACTTCCCCACCCCAACCATAA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | MT4 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779758 | ||
| mod ID: M6ASITE082663 | Click to Show/Hide the Full List | ||
| mod site | chr7:139570641-139570642:- | [6] | |
| Sequence | AACTCAGTAACGAAGTCGTTACTTAGCTCTTAGCTGTGAAA | ||
| Motif Score | 2.494845238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779759 | ||
| mod ID: M6ASITE082664 | Click to Show/Hide the Full List | ||
| mod site | chr7:139570660-139570661:- | [6] | |
| Sequence | TTGTAGTGATATCCAACCTAACTCAGTAACGAAGTCGTTAC | ||
| Motif Score | 2.590089286 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779760 | ||
| mod ID: M6ASITE082665 | Click to Show/Hide the Full List | ||
| mod site | chr7:139570964-139570965:- | [7] | |
| Sequence | GAAGTTTTCTCAGGCTTTGGACACTTCTGGGGATGGAGGTG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779761 | ||
| mod ID: M6ASITE082666 | Click to Show/Hide the Full List | ||
| mod site | chr7:139571049-139571050:- | [7] | |
| Sequence | TGACGCTGCACTTTGGAAAAACTCACACAGTTGAATTTCCA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779762 | ||
| mod ID: M6ASITE082667 | Click to Show/Hide the Full List | ||
| mod site | chr7:139571094-139571095:- | [7] | |
| Sequence | GTGGGCAGAAGGTTGGAGGGACACTTATGAGGGTGGCCGGG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; kidney | ||
| Seq Type List | m6A-seq; m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779763 | ||
| mod ID: M6ASITE082668 | Click to Show/Hide the Full List | ||
| mod site | chr7:139571147-139571148:- | [7] | |
| Sequence | ATTACCCAGGTCTGCTCAAGACATGATTTTGGTTTTGGTTT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779764 | ||
| mod ID: M6ASITE082669 | Click to Show/Hide the Full List | ||
| mod site | chr7:139571208-139571209:- | [7] | |
| Sequence | CCAGATCCCTCCCTCTGTAGACAACCACCAACCTCTGTTTG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779765 | ||
| mod ID: M6ASITE082670 | Click to Show/Hide the Full List | ||
| mod site | chr7:139571237-139571238:- | [6] | |
| Sequence | CCCTGGGCTGTTTTGCTCTAACGATCTTGCCAGATCCCTCC | ||
| Motif Score | 2.142029762 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779766 | ||
| mod ID: M6ASITE082671 | Click to Show/Hide the Full List | ||
| mod site | chr7:139571375-139571376:- | [7] | |
| Sequence | GTGAGGCCAGAGGTTTCCGGACTGTTGGCCTCGCCAGGCAG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779767 | ||
| mod ID: M6ASITE082672 | Click to Show/Hide the Full List | ||
| mod site | chr7:139571444-139571445:- | [7] | |
| Sequence | GTGGGCGGAGGCCCAGAGAGACTCCCCACCCTTCACCCCTG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779768 | ||
| mod ID: M6ASITE082673 | Click to Show/Hide the Full List | ||
| mod site | chr7:139571538-139571539:- | [7] | |
| Sequence | TCTCGAAAGAAATATGGGAGACAGATGCCCGGCGGGTGCGT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779769 | ||
| mod ID: M6ASITE082674 | Click to Show/Hide the Full List | ||
| mod site | chr7:139571724-139571725:- | [7] | |
| Sequence | AAGAAACTGAGGCTAGTCAGACACAATCTCAGCTCTTCTGT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779770 | ||
| mod ID: M6ASITE082675 | Click to Show/Hide the Full List | ||
| mod site | chr7:139571739-139571740:- | [7] | |
| Sequence | TTCCACTGGTACTCCAAGAAACTGAGGCTAGTCAGACACAA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779771 | ||
| mod ID: M6ASITE082676 | Click to Show/Hide the Full List | ||
| mod site | chr7:139571782-139571783:- | [6] | |
| Sequence | TCCTAGTGCCTTACCGAGCGACAGACGCGGCGTGAGGGTTT | ||
| Motif Score | 2.865571429 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779772 | ||
| mod ID: M6ASITE082677 | Click to Show/Hide the Full List | ||
| mod site | chr7:139571956-139571957:- | [8] | |
| Sequence | ATATTCCTCAATGTAATTGCACAAAAAAAAGCGATATAACA | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779773 | ||
| mod ID: M6ASITE082678 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572048-139572049:- | [8] | |
| Sequence | AGGGCTAGCGGTCGTCACAGACACCCTAGTAGAATGTAGCA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779774 | ||
| mod ID: M6ASITE082679 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572357-139572358:- | [9] | |
| Sequence | TCCAGAACTCGAAAGGGTCAACAGCCCTCCAGAATGTCGGG | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779775 | ||
| mod ID: M6ASITE082680 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572371-139572372:- | [7] | |
| Sequence | GATTTAGGGATTTTTCCAGAACTCGAAAGGGTCAACAGCCC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; H1B; hNPCs; fibroblasts; GM12878; LCLs; MM6; Huh7; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779776 | ||
| mod ID: M6ASITE082681 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572397-139572398:- | [7] | |
| Sequence | ATAGATTTTTAATTAATTGAACATAAGATTTAGGGATTTTT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; H1B; H1A; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779777 | ||
| mod ID: M6ASITE082682 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572437-139572438:- | [7] | |
| Sequence | ACGGAAGACTGCTGTCAGGGACACCTGATATGGAAATCAAA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; brain; A549; U2OS; H1B; H1A; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779778 | ||
| mod ID: M6ASITE082683 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572450-139572451:- | [7] | |
| Sequence | GGCAGCAAGTGATACGGAAGACTGCTGTCAGGGACACCTGA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1B; H1A; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779779 | ||
| mod ID: M6ASITE082684 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572494-139572495:- | [7] | |
| Sequence | GTTTTTGAAGAACTTTATGAACTTTTCAAAGATTATTTTCA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779780 | ||
| mod ID: M6ASITE082685 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572503-139572504:- | [7] | |
| Sequence | GGGGCCCCAGTTTTTGAAGAACTTTATGAACTTTTCAAAGA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779781 | ||
| mod ID: M6ASITE082686 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572527-139572528:- | [14] | |
| Sequence | GCAACAGGAAGATTCCAGAAACGGGGGGCCCCAGTTTTTGA | ||
| Motif Score | 2.179660714 | ||
| Cell/Tissue List | CD8T; A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779782 | ||
| mod ID: M6ASITE082687 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572565-139572566:- | [7] | |
| Sequence | AAGAACCCATTCTACGCCAAACTGGAAAGGAGAAGAGAGCA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779783 | ||
| mod ID: M6ASITE082688 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572581-139572582:- | [7] | |
| Sequence | ACAAAAAACCCAGCTCAAGAACCCATTCTACGCCAAACTGG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779784 | ||
| mod ID: M6ASITE082689 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572594-139572595:- | [7] | |
| Sequence | AAAAAAGTCAATAACAAAAAACCCAGCTCAAGAACCCATTC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779785 | ||
| mod ID: M6ASITE082690 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572687-139572688:- | [7] | |
| Sequence | GACGTAGAGCAGAGAAGAGAACATTTTTAAAAGGAAGGGAT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; kidney; A549; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; HEK293A-TOA; iSLK; MSC; TIME | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-REF-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779786 | ||
| mod ID: M6ASITE082691 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572722-139572723:- | [7] | |
| Sequence | GGGACACCAGTGAAACTTGAACCGGGAAGTGGGAGGACGTA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; A549; HEK293T; hNPCs; hESCs; fibroblasts; GM12878; Huh7; HEK293A-TOA; iSLK; MSC; TIME | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779787 | ||
| mod ID: M6ASITE082692 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572728-139572729:- | [7] | |
| Sequence | CGGGTCGGGACACCAGTGAAACTTGAACCGGGAAGTGGGAG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; A549; HEK293T; H1B; H1A; hNPCs; hESCs; fibroblasts; GM12878; Huh7; HEK293A-TOA; iSLK; MSC; TIME | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779788 | ||
| mod ID: M6ASITE082693 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572739-139572740:- | [7] | |
| Sequence | GGGCAGGGGGACGGGTCGGGACACCAGTGAAACTTGAACCG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; A549; HEK293T; H1B; H1A; hNPCs; hESCs; fibroblasts; GM12878; Huh7; HEK293A-TOA; iSLK; MSC; TIME | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779789 | ||
| mod ID: M6ASITE082694 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572802-139572803:- | [7] | |
| Sequence | ACTGAAGATGCCGCACACAAACAATGCAAACGGGGCAGGGG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; A549; HepG2; H1A; H1B; hNPCs; hESCs; HEK293T; fibroblasts; GM12878; MT4; Huh7; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779790 | ||
| mod ID: M6ASITE082695 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572844-139572845:- | [7] | |
| Sequence | AGGAGGGAGGCGCTCCTGGGACCGTGGGCGCTGGCCTTTTA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; A549; HepG2; H1B; H1A; hESCs; HEK293T; fibroblasts; GM12878; Huh7; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779791 | ||
| mod ID: M6ASITE082696 | Click to Show/Hide the Full List | ||
| mod site | chr7:139572925-139572926:- | [7] | |
| Sequence | ACCAGTACCCTTACATATAAACACTGGAGGGGAGGGAGGGA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HepG2; A549; hESCs; HEK293T; fibroblasts; GM12878; MT4; Huh7; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; NB4; MM6 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779792 | ||
| mod ID: M6ASITE082697 | Click to Show/Hide the Full List | ||
| mod site | chr7:139573012-139573013:- | [7] | |
| Sequence | CAGCCCAATTTGCCCACCAGACCTACATCAGCGCCTCGCCA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; U2OS; A549; GM12878; MT4; MM6; Huh7; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779793 | ||
| mod ID: M6ASITE082698 | Click to Show/Hide the Full List | ||
| mod site | chr7:139573374-139573375:- | [7] | |
| Sequence | TCAGCAGCACATCACCACGGACCGCACTGGGAGCCACCGAA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; MT4; Huh7; iSLK; MSC; TIME | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779794 | ||
| mod ID: M6ASITE082699 | Click to Show/Hide the Full List | ||
| mod site | chr7:139583861-139583862:- | [7] | |
| Sequence | TCATCGTGCCACCCCTGAAAACCCAGGCCAGCGAAGTATTG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779795 | ||
| mod ID: M6ASITE082700 | Click to Show/Hide the Full List | ||
| mod site | chr7:139583885-139583886:- | [7] | |
| Sequence | ACTGCACGGGGAACCCCCGAACCATCATCGTGCCACCCCTG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779796 | ||
| mod ID: M6ASITE082701 | Click to Show/Hide the Full List | ||
| mod site | chr7:139583893-139583894:- | [7] | |
| Sequence | GGAGAATCACTGCACGGGGAACCCCCGAACCATCATCGTGC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779797 | ||
| mod ID: M6ASITE082702 | Click to Show/Hide the Full List | ||
| mod site | chr7:139596730-139596731:- | [7] | |
| Sequence | GACGAGGAGGAGGAACAGAAACACGCCCCCACCAGGTGAGG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; MT4; Huh7; CD4T; peripheral-blood; GSC-11; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779798 | ||
| mod ID: M6ASITE082703 | Click to Show/Hide the Full List | ||
| mod site | chr7:139596736-139596737:- | [7] | |
| Sequence | GACACGGACGAGGAGGAGGAACAGAAACACGCCCCCACCAG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; MT4; Huh7; CD4T; peripheral-blood; GSC-11; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779799 | ||
| mod ID: M6ASITE082704 | Click to Show/Hide the Full List | ||
| mod site | chr7:139596813-139596814:- | [7] | |
| Sequence | CCCGGGAACGGCAGCGGCAGACAATTGTCATTCCCGACACT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; MT4; Huh7; peripheral-blood; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779800 | ||
| mod ID: M6ASITE082705 | Click to Show/Hide the Full List | ||
| mod site | chr7:139596923-139596924:- | [7] | |
| Sequence | ATCCAAGCGTGTCAAGGAGAACACACCTCCCCGCTGTGCCA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779801 | ||
| mod ID: M6ASITE082706 | Click to Show/Hide the Full List | ||
| mod site | chr7:139600511-139600512:- | [9] | |
| Sequence | CATGTGACCCTTCCAGCAGCACAGCCCTTAAATGTGGGTGT | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | brain | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779802 | ||
| mod ID: M6ASITE082707 | Click to Show/Hide the Full List | ||
| mod site | chr7:139604086-139604087:- | [7] | |
| Sequence | AGGCACCCAGCAGCTGGCGGACTGGAGGTAAGCAGGAAACC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779803 | ||
| mod ID: M6ASITE082708 | Click to Show/Hide the Full List | ||
| mod site | chr7:139604114-139604115:- | [7] | |
| Sequence | ATGCCACCGTGATTCCCGAGACCATGGCAGGCACCCAGCAG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779804 | ||
| mod ID: M6ASITE082709 | Click to Show/Hide the Full List | ||
| mod site | chr7:139604204-139604205:- | [7] | |
| Sequence | AGCAGGCTTGGCCAAGTGGGACCCAGCAGATCCTGCTTCCC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779805 | ||
| mod ID: M6ASITE082710 | Click to Show/Hide the Full List | ||
| mod site | chr7:139614354-139614355:- | [7] | |
| Sequence | GCATGCCCCTGCAGACAGGAACAGCCCAGATTTGTGCCCGG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779806 | ||
| mod ID: M6ASITE082711 | Click to Show/Hide the Full List | ||
| mod site | chr7:139614360-139614361:- | [7] | |
| Sequence | AGCGGAGCATGCCCCTGCAGACAGGAACAGCCCAGATTTGT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779807 | ||
| mod ID: M6ASITE082712 | Click to Show/Hide the Full List | ||
| mod site | chr7:139614434-139614435:- | [7] | |
| Sequence | TCCCGAAGTCTCCATACTAAACTACCCATCTACACTCTACC | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; LCLs; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779808 | ||
| mod ID: M6ASITE082713 | Click to Show/Hide the Full List | ||
| mod site | chr7:139620486-139620487:- | [7] | |
| Sequence | ACACGGTGAACCAGAGCAAAACCCCTTTCATCACGCACGTG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779809 | ||
| mod ID: M6ASITE082714 | Click to Show/Hide the Full List | ||
| mod site | chr7:139620497-139620498:- | [7] | |
| Sequence | GAATATGTATGACACGGTGAACCAGAGCAAAACCCCTTTCA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779810 | ||
| mod ID: M6ASITE082715 | Click to Show/Hide the Full List | ||
| mod site | chr7:139620542-139620543:- | [7] | |
| Sequence | CGTCAAATCATGTTTCCAGAACATGGAGATCTGCAAGCGTC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779811 | ||
| mod ID: M6ASITE082716 | Click to Show/Hide the Full List | ||
| mod site | chr7:139626651-139626652:- | [7] | |
| Sequence | CACTCCAATCGAAACCCTGAACCATCCCTTTGTCACCATGA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779812 | ||
| mod ID: M6ASITE082717 | Click to Show/Hide the Full List | ||
| mod site | chr7:139626658-139626659:- | [7] | |
| Sequence | AGAGAATCACTCCAATCGAAACCCTGAACCATCCCTTTGTC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779813 | ||
| mod ID: M6ASITE082718 | Click to Show/Hide the Full List | ||
| mod site | chr7:139626681-139626682:- | [8] | |
| Sequence | GATGCTGACCATTGATGCTGACAAGAGAATCACTCCAATCG | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779814 | ||
| mod ID: M6ASITE082719 | Click to Show/Hide the Full List | ||
| mod site | chr7:139626753-139626754:- | [8] | |
| Sequence | GACAGATTTGGAAGGGAGCGACATGTTGGTAGAAAAGGCTG | ||
| Motif Score | 2.865571429 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779815 | ||
| mod ID: M6ASITE082720 | Click to Show/Hide the Full List | ||
| mod site | chr7:139626780-139626781:- | [7] | |
| Sequence | CCTTACTTGCGTCCAGGTGAACATGACGACAGATTTGGAAG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779816 | ||
| mod ID: M6ASITE082721 | Click to Show/Hide the Full List | ||
| mod site | chr7:139629014-139629015:- | [7] | |
| Sequence | CAGATGACCATGAAGCAGAGACAGGGATTAAGTCAAAAGAA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779817 | ||
| mod ID: M6ASITE082722 | Click to Show/Hide the Full List | ||
| mod site | chr7:139631170-139631171:- | [7] | |
| Sequence | TCACCATATCCTTTGTGGAGACTGAAGGTAGGAAGAGCGTC | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779818 | ||
| mod ID: M6ASITE082723 | Click to Show/Hide the Full List | ||
| mod site | chr7:139631192-139631193:- | [7] | |
| Sequence | GTTTTTCAACCGTGACACGGACTCACCATATCCTTTGTGGA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779819 | ||
| mod ID: M6ASITE082724 | Click to Show/Hide the Full List | ||
| mod site | chr7:139631220-139631221:- | [7] | |
| Sequence | TATTAAGCGCCGGGACAAAGACAACTAGGTTTTTCAACCGT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779820 | ||
| mod ID: M6ASITE082725 | Click to Show/Hide the Full List | ||
| mod site | chr7:139631226-139631227:- | [7] | |
| Sequence | AATATTTATTAAGCGCCGGGACAAAGACAACTAGGTTTTTC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779821 | ||
| mod ID: M6ASITE082726 | Click to Show/Hide the Full List | ||
| mod site | chr7:139631265-139631266:- | [7] | |
| Sequence | AGATTCGGTATATTTCACAAACACAGGGTTTGCCTGCTGAA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779822 | ||
| mod ID: M6ASITE082727 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716023-139716024:- | [7] | |
| Sequence | ATGCTGGTGGATCCATCTAGACAACCATACAGAGTCAAGGT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; A549; GM12878; H1299; Huh7; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779823 | ||
| mod ID: M6ASITE082728 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716048-139716049:- | [7] | |
| Sequence | CGCTGACCTCAAACCAGAAAACATCATGCTGGTGGATCCAT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; fibroblasts; GM12878; LCLs; H1299; Huh7; Jurkat; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779824 | ||
| mod ID: M6ASITE082729 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716056-139716057:- | [7] | |
| Sequence | CTTATCCACGCTGACCTCAAACCAGAAAACATCATGCTGGT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; fibroblasts; GM12878; LCLs; H1299; Huh7; Jurkat; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779825 | ||
| mod ID: M6ASITE082730 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716092-139716093:- | [7] | |
| Sequence | GTAGCCACAGCCCTGATGAAACTCAAAAGCCTAGGTCTTAT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1B; hNPCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779826 | ||
| mod ID: M6ASITE082731 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716162-139716163:- | [7] | |
| Sequence | CTATGACTTTCTGAAGCAAAACAAGTTTAGCCCCTTGCCCC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; hESC-HEK293T; U2OS; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779827 | ||
| mod ID: M6ASITE082732 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716177-139716178:- | [14] | |
| Sequence | GTTGGAGCAGAACCTCTATGACTTTCTGAAGCAAAACAAGT | ||
| Motif Score | 3.28175 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779828 | ||
| mod ID: M6ASITE082733 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716186-139716187:- | [7] | |
| Sequence | CTTCGAGATGTTGGAGCAGAACCTCTATGACTTTCTGAAGC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779829 | ||
| mod ID: M6ASITE082734 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716219-139716220:- | [8] | |
| Sequence | ATGCTTCCAGCACAAGAACCACACGTGCTTGGTCTTCGAGA | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779830 | ||
| mod ID: M6ASITE082735 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716222-139716223:- | [7] | |
| Sequence | CGAATGCTTCCAGCACAAGAACCACACGTGCTTGGTCTTCG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779831 | ||
| mod ID: M6ASITE082736 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716228-139716229:- | [8] | |
| Sequence | GGCCTACGAATGCTTCCAGCACAAGAACCACACGTGCTTGG | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779832 | ||
| mod ID: M6ASITE082737 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716264-139716265:- | [14] | |
| Sequence | GAGCACGGAGAGTGCCGATGACTATAACTTCGTCCGGGCCT | ||
| Motif Score | 3.28175 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779833 | ||
| mod ID: M6ASITE082738 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716320-139716321:- | [8] | |
| Sequence | AACCACCCATCCTATGCCCGACAAGGTCAGATTGAAGTGAG | ||
| Motif Score | 2.865571429 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779834 | ||
| mod ID: M6ASITE082739 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716339-139716340:- | [7] | |
| Sequence | AGCCATCAAGATCCTGAAGAACCACCCATCCTATGCCCGAC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; MT4; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779835 | ||
| mod ID: M6ASITE082740 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716409-139716410:- | [14] | |
| Sequence | TAGAGTTCTTGGGCCGAGGGACGTTTGGGCAAGTGGTCAAG | ||
| Motif Score | 3.616982143 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779836 | ||
| mod ID: M6ASITE082741 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716510-139716511:- | [7] | |
| Sequence | GTCTACTGCCACCTCCAAAAACAGCGGCTCCAACAGCGAGG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; fibroblasts; A549; GM12878; LCLs; MT4; H1299; Huh7; Jurkat; peripheral-blood; iSLK; MSC; TIME; TREX; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779837 | ||
| mod ID: M6ASITE082742 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716615-139716616:- | [7] | |
| Sequence | TAAGAGCGAGGAGATCGAGAACACAAGCAGCGTGCAGATCA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; hESC-HEK293T; hNPCs; fibroblasts; A549; GM12878; MT4; H1299; Huh7; Jurkat; peripheral-blood; iSLK; MSC; TIME; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779838 | ||
| mod ID: M6ASITE082743 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716644-139716645:- | [7] | |
| Sequence | GATACCTACCAAAAATGTGGACTCAAGCGTAAGAGCGAGGA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; U2OS; hNPCs; fibroblasts; A549; CD8T; MT4; Huh7; Jurkat; peripheral-blood; iSLK; MSC; TIME; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779839 | ||
| mod ID: M6ASITE082744 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716704-139716705:- | [7] | |
| Sequence | ACCGGGCAAGTCCTCGGCGGACCACACAACCTAATGCGTCG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; Brain; fibroblasts; A549; MT4; Huh7; Jurkat; peripheral-blood; iSLK; MSC; TIME; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779840 | ||
| mod ID: M6ASITE082745 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716759-139716760:- | [8] | |
| Sequence | CTTCCCAGGAAGCACCGGGCACATCGTGGTCACCTCAGCAA | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779841 | ||
| mod ID: M6ASITE082746 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716787-139716788:- | [7] | |
| Sequence | CAAGCCTACCTTACGAGCAGACCATCGTCTTCCCAGGAAGC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; Brain; fibroblasts; A549; MT4; MM6; Huh7; Jurkat; peripheral-blood; iSLK; MSC; TIME; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779842 | ||
| mod ID: M6ASITE082747 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716810-139716811:- | [7] | |
| Sequence | CACCTCCTTGCCGGTCCCAAACCCAAGCCTACCTTACGAGC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; Brain; fibroblasts; A549; MT4; MM6; Huh7; Jurkat; iSLK; MSC; TIME; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000342645.7; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779843 | ||
| mod ID: M6ASITE082748 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716867-139716868:- | [7] | |
| Sequence | AGTGTATAGCCAGAGCAAGAACATCCCCCTGTCGCAGCCAG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; hESCs; fibroblasts; A549; LCLs; MT4; MM6; Huh7; iSLK; MSC; TIME; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000342645.7; ENST00000428878.6; ENST00000406875.8 | ||
| External Link | RMBase: m6A_site_779844 | ||
| mod ID: M6ASITE082749 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716894-139716895:- | [9] | |
| Sequence | CATGACTGGGTACGGCTCCCACAGCAAAGTGTATAGCCAGA | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | brain; kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779845 | ||
| mod ID: M6ASITE082750 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716915-139716916:- | [7] | |
| Sequence | AGAGCCGAGTTCCAACTGGGACATGACTGGGTACGGCTCCC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; hESC-HEK293T; hESCs; fibroblasts; A549; CD8T; MT4; MM6; Huh7; iSLK; MSC; TIME; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000428878.6; ENST00000406875.8; ENST00000342645.7 | ||
| External Link | RMBase: m6A_site_779846 | ||
| mod ID: M6ASITE082751 | Click to Show/Hide the Full List | ||
| mod site | chr7:139716944-139716945:- | [7] | |
| Sequence | GCCTTCTGTAGTGTGAAGAAACTGAAAATAGAGCCGAGTTC | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; fibroblasts; A549; MT4; MM6; Huh7; iSLK; MSC; TIME | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000342645.7; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779847 | ||
| mod ID: M6ASITE082752 | Click to Show/Hide the Full List | ||
| mod site | chr7:139777636-139777637:- | [7] | |
| Sequence | CCCGTTGCCATGAACCGCGGACACCCCGGCCCCGATGGCCC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779848 | ||
| mod ID: M6ASITE082753 | Click to Show/Hide the Full List | ||
| mod site | chr7:139777643-139777644:- | [7] | |
| Sequence | TCCCGTCCCCGTTGCCATGAACCGCGGACACCCCGGCCCCG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779849 | ||
| mod ID: M6ASITE082754 | Click to Show/Hide the Full List | ||
| mod site | chr7:139777724-139777725:- | [7] | |
| Sequence | CGGCTGCGCCGAGAGCGGAGACACAGGCTCAAGATGGCAGA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000406875.8; ENST00000428878.6 | ||
| External Link | RMBase: m6A_site_779850 | ||
References