m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00313)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
RPS6KB1
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Browse Drug
Methyltransferase-like 3 (METTL3) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL3 | ||
| Cell Line | CT26 cell line | Mus musculus |
|
Treatment: METTL3 knockout CT26 cells
Control: CT26 cells
|
GSE142589 | |
| Regulation |
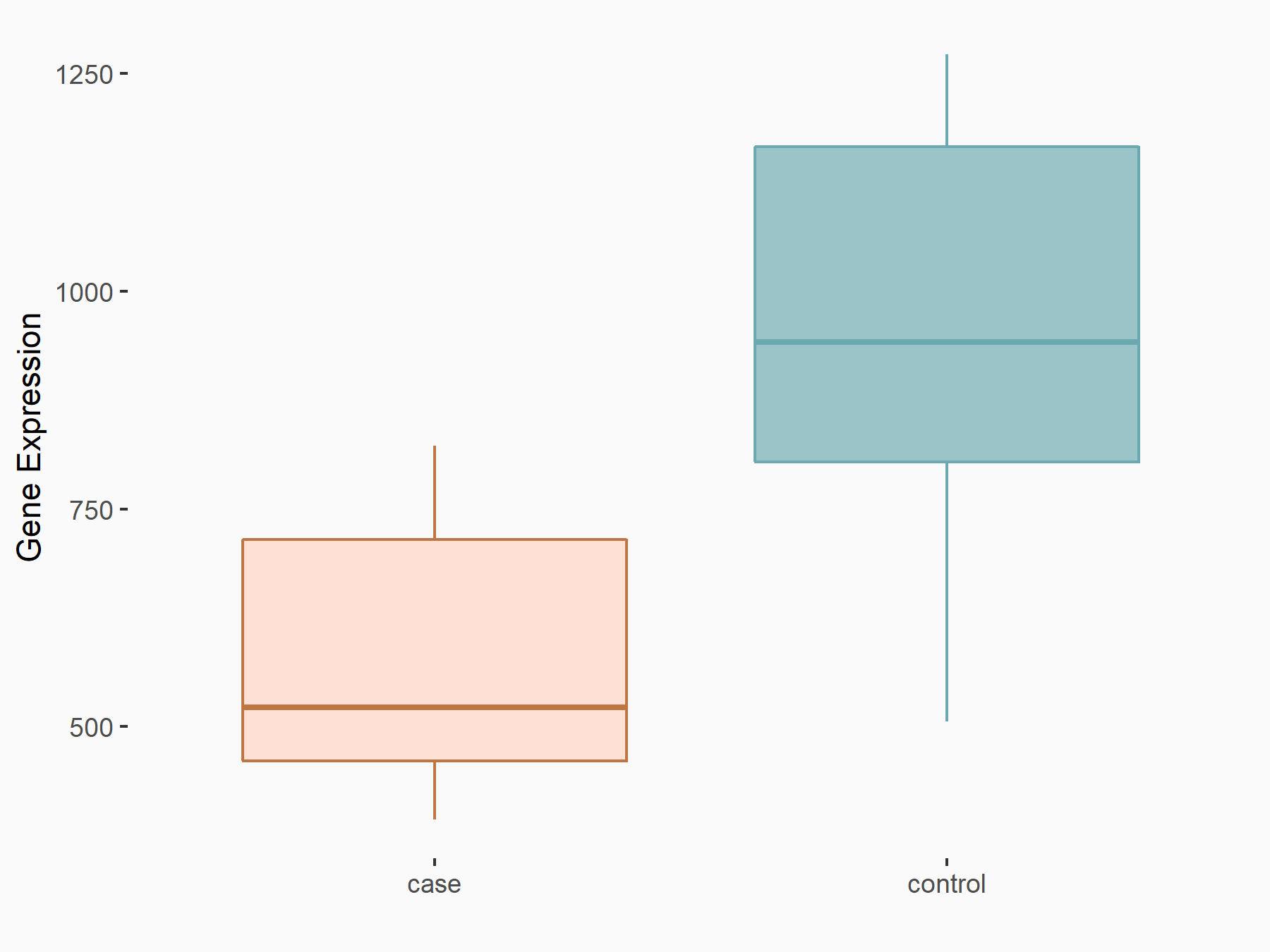 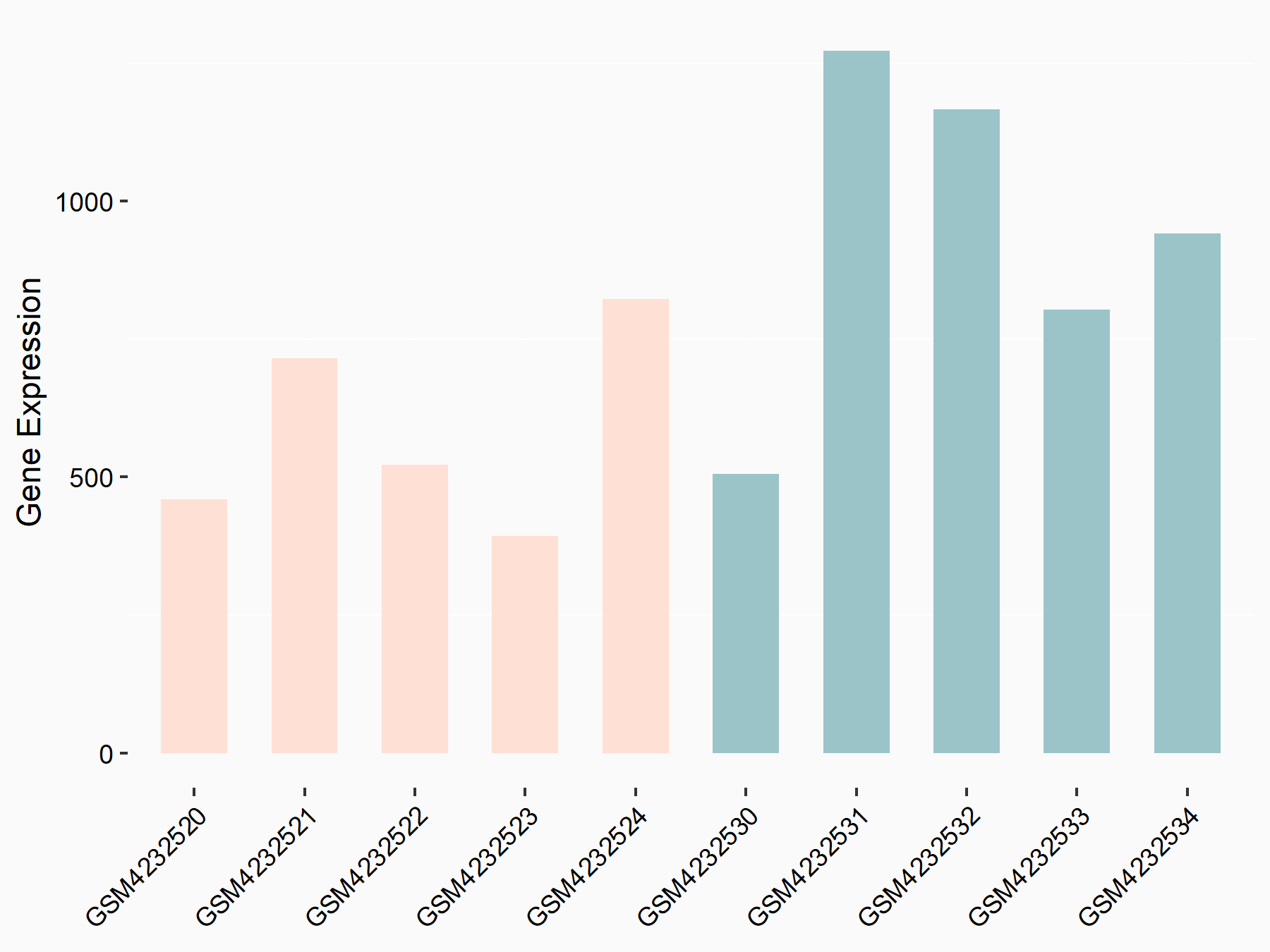 |
logFC: -6.86E-01 p-value: 2.67E-02 |
| More Results | Click to View More RNA-seq Results | |
| Representative RIP-seq result supporting the interaction between RPS6KB1 and the regulator | ||
| Cell Line | MDA-MB-231 | Homo sapiens |
| Regulation | logFC: 1.70E+00 | GSE60213 |
| In total 2 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | Down-regulation of METTL3 inhibits the proliferation and mobility of human gastric cancer cells and leads to inactivation of the AKT signaling pathway, suggesting that METTL3 is a potential target for the treatment of human gastric cancer. METTL3 knockdown decreased Bcl2 and increased Bax and active Caspase-3 in gastric cancer cells, which suggested the apoptotic pathway was activated. METTL3 led to inactivation of the AKT signaling pathway in human gastric cancer cells, including decreased phosphorylation levels of AKT and expression of down-stream effectors Ribosomal protein S6 kinase beta-1 (RPS6KB1/p70S6K) and Cyclin D1. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | ||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Cell Process | Cell proliferation | |||
| Cell migration | ||||
| Cell invasion | ||||
| In-vitro Model | AGS | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 |
| MKN45 | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
| Experiment 2 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | METTL3 promotes the progression of retinoblastoma through PI3K/AKT/mTOR pathways in vitro and in vivo. METTL3 has an impact on the PI3K-AKT-mTOR-Ribosomal protein S6 kinase beta-1 (RPS6KB1/p70S6K)/4EBP1 pathway. The cell proliferation results show that the stimulatory function of METTL3 is lost after rapamycin treatment. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Retinoblastoma | ICD-11: 2D02.2 | ||
| Responsed Drug | Rapamycin | Approved | ||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| mTOR signaling pathway | hsa04150 | |||
| Cell Process | Cell proliferation | |||
| Cell migration | ||||
| Cell invasion | ||||
| Cell apoptosis | ||||
| In-vitro Model | WERI-Rb-1 | Retinoblastoma | Homo sapiens | CVCL_1792 |
| Y-79 | Retinoblastoma | Homo sapiens | CVCL_1893 | |
| In-vivo Model | To establish a subcutaneous tumour model in nude mice, 2 × 107 Y79 cells (METTL3 knockdown group: shNC, shRNA1 and shRNA2; METTL3 up-regulated group: NC and METLL3) were resuspended in 1 mL of pre-cooled PBS, and 200 uL of the cell suspension was injected subcutaneously into the left side of the armpit to investigate tumour growth (4 × 106 per mouse). | |||
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | Down-regulation of METTL3 inhibits the proliferation and mobility of human gastric cancer cells and leads to inactivation of the AKT signaling pathway, suggesting that METTL3 is a potential target for the treatment of human gastric cancer. METTL3 knockdown decreased Bcl2 and increased Bax and active Caspase-3 in gastric cancer cells, which suggested the apoptotic pathway was activated. METTL3 led to inactivation of the AKT signaling pathway in human gastric cancer cells, including decreased phosphorylation levels of AKT and expression of down-stream effectors Ribosomal protein S6 kinase beta-1 (RPS6KB1/p70S6K) and Cyclin D1. | |||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Cell Process | Cell proliferation | |||
| Cell migration | ||||
| Cell invasion | ||||
| In-vitro Model | AGS | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 |
| MKN45 | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
Retina cancer [ICD-11: 2D02]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [2] | |||
| Response Summary | METTL3 promotes the progression of retinoblastoma through PI3K/AKT/mTOR pathways in vitro and in vivo. METTL3 has an impact on the PI3K-AKT-mTOR-Ribosomal protein S6 kinase beta-1 (RPS6KB1/p70S6K)/4EBP1 pathway. The cell proliferation results show that the stimulatory function of METTL3 is lost after rapamycin treatment. | |||
| Responsed Disease | Retinoblastoma [ICD-11: 2D02.2] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Drug | Rapamycin | Approved | ||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| mTOR signaling pathway | hsa04150 | |||
| Cell Process | Cell proliferation | |||
| Cell migration | ||||
| Cell invasion | ||||
| Cell apoptosis | ||||
| In-vitro Model | WERI-Rb-1 | Retinoblastoma | Homo sapiens | CVCL_1792 |
| Y-79 | Retinoblastoma | Homo sapiens | CVCL_1893 | |
| In-vivo Model | To establish a subcutaneous tumour model in nude mice, 2 × 107 Y79 cells (METTL3 knockdown group: shNC, shRNA1 and shRNA2; METTL3 up-regulated group: NC and METLL3) were resuspended in 1 mL of pre-cooled PBS, and 200 uL of the cell suspension was injected subcutaneously into the left side of the armpit to investigate tumour growth (4 × 106 per mouse). | |||
Rapamycin
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response | [2] | |||
| Response Summary | METTL3 promotes the progression of retinoblastoma through PI3K/AKT/mTOR pathways in vitro and in vivo. METTL3 has an impact on the PI3K-AKT-mTOR-Ribosomal protein S6 kinase beta-1 (RPS6KB1/p70S6K)/4EBP1 pathway. The cell proliferation results show that the stimulatory function of METTL3 is lost after rapamycin treatment. | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Disease | Retinoblastoma | ICD-11: 2D02.2 | ||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| mTOR signaling pathway | hsa04150 | |||
| Cell Process | Cell proliferation | |||
| Cell migration | ||||
| Cell invasion | ||||
| Cell apoptosis | ||||
| In-vitro Model | WERI-Rb-1 | Retinoblastoma | Homo sapiens | CVCL_1792 |
| Y-79 | Retinoblastoma | Homo sapiens | CVCL_1893 | |
| In-vivo Model | To establish a subcutaneous tumour model in nude mice, 2 × 107 Y79 cells (METTL3 knockdown group: shNC, shRNA1 and shRNA2; METTL3 up-regulated group: NC and METLL3) were resuspended in 1 mL of pre-cooled PBS, and 200 uL of the cell suspension was injected subcutaneously into the left side of the armpit to investigate tumour growth (4 × 106 per mouse). | |||
Full List of Crosstalk(s) between m6A Modification and Epigenetic Regulation Related to This Regulator
Histone modification
m6A Regulator: Methyltransferase-like 3 (METTL3)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT03638 | ||
| Epigenetic Regulator | Histone-lysine N-methyltransferase EZH2 (EZH2) | |
| Regulated Target | Histone H3 lysine 27 trimethylation (H3K27me3) | |
| Crosstalk relationship | Histone modification → m6A | |
| Disease | Gastric cancer | |
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00313)
| In total 3 m6A sequence/site(s) in this target gene | |||
| mod ID: A2ISITE008339 | Click to Show/Hide the Full List | ||
| mod site | chr17:59894813-59894814:+ | [3] | |
| Sequence | AGGGTTTCACCATATTGGCCAGGCTGTTCTCGGACTCCTGA | ||
| Transcript ID List | ENST00000406116.7; ENST00000225577.9; ENST00000393021.7; ENST00000443572.6; ENST00000472940.5; ENST00000477179.5; ENST00000489824.1 | ||
| External Link | RMBase: RNA-editing_site_59651 | ||
| mod ID: A2ISITE008340 | Click to Show/Hide the Full List | ||
| mod site | chr17:59895906-59895907:+ | [3] | |
| Sequence | CCTCTCAAAGTGCTGGGATTACAGGCGTGAGCCACTGCATC | ||
| Transcript ID List | ENST00000443572.6; ENST00000393021.7; ENST00000406116.7; ENST00000477179.5; ENST00000225577.9; ENST00000489824.1; ENST00000472940.5 | ||
| External Link | RMBase: RNA-editing_site_59652 | ||
| mod ID: A2ISITE008341 | Click to Show/Hide the Full List | ||
| mod site | chr17:59936603-59936604:+ | [4] | |
| Sequence | GCCTGTAATCCCCAAACTTTAAAGGGCCAAGGTGGGAGGAT | ||
| Transcript ID List | rmsk_4733409; ENST00000406116.7; ENST00000443572.6; ENST00000225577.9; ENST00000393021.7; ENST00000472940.5 | ||
| External Link | RMBase: RNA-editing_site_59653 | ||
N6-methyladenosine (m6A)
| In total 87 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE034512 | Click to Show/Hide the Full List | ||
| mod site | chr17:59893060-59893061:+ | [5] | |
| Sequence | CCACTGTTTGGCTTCACGGAACCCTGTACGCATGCTCCTAC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_374932 | ||
| mod ID: M6ASITE034513 | Click to Show/Hide the Full List | ||
| mod site | chr17:59893086-59893087:+ | [5] | |
| Sequence | TACGCATGCTCCTACGCTGAACTTTAGGAGCCAGTCTAAGG | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; A549; HEK293A-TOA; iSLK | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000393021.7; ENST00000472940.5 | ||
| External Link | RMBase: m6A_site_374933 | ||
| mod ID: M6ASITE034514 | Click to Show/Hide the Full List | ||
| mod site | chr17:59893227-59893228:+ | [5] | |
| Sequence | CGGCTTTTACCCAGCCCCGGACTTCCGAGACAGGGAAGCTG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; A549; U2OS; MT4; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293T; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000477179.5; ENST00000489824.1; ENST00000406116.7; ENST00000472940.5; ENST00000393021.7; ENST00000225577.9; ENST00000443572.6 | ||
| External Link | RMBase: m6A_site_374934 | ||
| mod ID: M6ASITE034515 | Click to Show/Hide the Full List | ||
| mod site | chr17:59893236-59893237:+ | [5] | |
| Sequence | CCCAGCCCCGGACTTCCGAGACAGGGAAGCTGAGGACATGG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; A549; U2OS; MT4; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293T; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000477179.5; ENST00000443572.6; ENST00000489824.1; ENST00000472940.5; ENST00000393021.7; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374935 | ||
| mod ID: M6ASITE034516 | Click to Show/Hide the Full List | ||
| mod site | chr17:59893251-59893252:+ | [5] | |
| Sequence | CCGAGACAGGGAAGCTGAGGACATGGCAGGAGTGTTTGACA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; A549; U2OS; MT4; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293T; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000477179.5; ENST00000472940.5; ENST00000393021.7; ENST00000225577.9; ENST00000489824.1; ENST00000443572.6; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374936 | ||
| mod ID: M6ASITE034517 | Click to Show/Hide the Full List | ||
| mod site | chr17:59893275-59893276:+ | [5] | |
| Sequence | GGCAGGAGTGTTTGACATAGACCTGGACCAGCCAGAGGACG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; A549; U2OS; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293T; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000393021.7; ENST00000406116.7; ENST00000477179.5; ENST00000443572.6; ENST00000489824.1; ENST00000472940.5 | ||
| External Link | RMBase: m6A_site_374937 | ||
| mod ID: M6ASITE034518 | Click to Show/Hide the Full List | ||
| mod site | chr17:59893281-59893282:+ | [5] | |
| Sequence | AGTGTTTGACATAGACCTGGACCAGCCAGAGGACGCGGGCT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; A549; U2OS; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293T; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000393021.7; ENST00000489824.1; ENST00000477179.5; ENST00000472940.5; ENST00000406116.7; ENST00000443572.6; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_374938 | ||
| mod ID: M6ASITE034519 | Click to Show/Hide the Full List | ||
| mod site | chr17:59893828-59893829:+ | [5] | |
| Sequence | GTTAGAAGTGACAGCTGAAAACTTCTGTCGGGAGTAGCACT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; iSLK; TREX; MSC; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000472940.5; ENST00000225577.9; ENST00000406116.7; ENST00000393021.7; ENST00000477179.5; ENST00000443572.6; ENST00000489824.1 | ||
| External Link | RMBase: m6A_site_374939 | ||
| mod ID: M6ASITE034520 | Click to Show/Hide the Full List | ||
| mod site | chr17:59910583-59910584:+ | [6] | |
| Sequence | TCAGTTAAATGAAAGCATGGACCATGGGGGAGTTGGACCAT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HepG2; U2OS; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406116.7; ENST00000393021.7; ENST00000472940.5; ENST00000443572.6; ENST00000592726.1; ENST00000225577.9; ENST00000489824.1; ENST00000477179.5 | ||
| External Link | RMBase: m6A_site_374940 | ||
| mod ID: M6ASITE034521 | Click to Show/Hide the Full List | ||
| mod site | chr17:59910599-59910600:+ | [6] | |
| Sequence | ATGGACCATGGGGGAGTTGGACCATATGAACTGTAAGTTTA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HepG2; U2OS; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000472940.5; ENST00000393021.7; ENST00000477179.5; ENST00000489824.1; ENST00000406116.7; ENST00000443572.6; ENST00000592726.1 | ||
| External Link | RMBase: m6A_site_374941 | ||
| mod ID: M6ASITE034522 | Click to Show/Hide the Full List | ||
| mod site | chr17:59910608-59910609:+ | [5] | |
| Sequence | GGGGGAGTTGGACCATATGAACTGTAAGTTTATATGAAGAG | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; U2OS; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000477179.5; ENST00000393021.7; ENST00000592726.1; ENST00000489824.1; ENST00000406116.7; ENST00000472940.5; ENST00000443572.6 | ||
| External Link | RMBase: m6A_site_374942 | ||
| mod ID: M6ASITE034523 | Click to Show/Hide the Full List | ||
| mod site | chr17:59912692-59912693:+ | [5] | |
| Sequence | TTTCTTCCCAGTGGCATGGAACATTGTGAGAAATTTGAAAT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; U2OS; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000477179.5; ENST00000225577.9; ENST00000443572.6; ENST00000393021.7; ENST00000472940.5; ENST00000489824.1; ENST00000406116.7; ENST00000592726.1 | ||
| External Link | RMBase: m6A_site_374943 | ||
| mod ID: M6ASITE034524 | Click to Show/Hide the Full List | ||
| mod site | chr17:59912720-59912721:+ | [5] | |
| Sequence | AGAAATTTGAAATCTCAGAAACTAGTGTGAACAGAGGGCCA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; U2OS; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000443572.6; ENST00000489824.1; ENST00000393021.7; ENST00000472940.5; ENST00000406116.7; ENST00000592726.1; ENST00000477179.5 | ||
| External Link | RMBase: m6A_site_374944 | ||
| mod ID: M6ASITE034525 | Click to Show/Hide the Full List | ||
| mod site | chr17:59912730-59912731:+ | [5] | |
| Sequence | AATCTCAGAAACTAGTGTGAACAGAGGGCCAGAAAAAATCA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; U2OS; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406116.7; ENST00000592726.1; ENST00000472940.5; ENST00000393021.7; ENST00000489824.1; ENST00000477179.5; ENST00000225577.9; ENST00000443572.6 | ||
| External Link | RMBase: m6A_site_374945 | ||
| mod ID: M6ASITE034526 | Click to Show/Hide the Full List | ||
| mod site | chr17:59912752-59912753:+ | [5] | |
| Sequence | AGAGGGCCAGAAAAAATCAGACCAGAATGTTTTGAGCTACT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; U2OS; Huh7 | ||
| Seq Type List | m6A-seq; DART-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000592726.1; ENST00000393021.7; ENST00000477179.5; ENST00000225577.9; ENST00000443572.6; ENST00000472940.5; ENST00000489824.1; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374946 | ||
| mod ID: M6ASITE034527 | Click to Show/Hide the Full List | ||
| mod site | chr17:59914655-59914656:+ | [7] | |
| Sequence | TTTTTCAAGTACGAAAAGTAACAGGAGCAAATACTGGGAAA | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000489824.1; ENST00000472940.5; ENST00000592726.1; ENST00000443572.6; ENST00000225577.9; ENST00000393021.7; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374947 | ||
| mod ID: M6ASITE034528 | Click to Show/Hide the Full List | ||
| mod site | chr17:59926470-59926471:+ | [7] | |
| Sequence | ATGCTAAAGATACAGCTCATACAAAAGCAGAACGGAATATT | ||
| Motif Score | 2.110482143 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000443572.6; ENST00000225577.9; ENST00000472940.5; ENST00000489824.1; ENST00000406116.7; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_374948 | ||
| mod ID: M6ASITE034529 | Click to Show/Hide the Full List | ||
| mod site | chr17:59926553-59926554:+ | [5] | |
| Sequence | GCCTTTCAGACTGGTGGAAAACTCTACCTCATCCTTGAGTA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000443572.6; ENST00000472940.5; ENST00000489824.1; ENST00000393021.7; ENST00000406116.7; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_374949 | ||
| mod ID: M6ASITE034530 | Click to Show/Hide the Full List | ||
| mod site | chr17:59930123-59930124:+ | [5] | |
| Sequence | TTTTTCTTTACAGGAGGAGAACTATTTATGCAGTTAGAAAG | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000472940.5; ENST00000489824.1; ENST00000443572.6; ENST00000406116.7; ENST00000393021.7; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_374950 | ||
| mod ID: M6ASITE034531 | Click to Show/Hide the Full List | ||
| mod site | chr17:59930164-59930165:+ | [5] | |
| Sequence | AGAGGGAATATTTATGGAAGACACTGCCTGGTAAGTGAACT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000489824.1; ENST00000393021.7; ENST00000406116.7; ENST00000443572.6; ENST00000225577.9; ENST00000472940.5 | ||
| External Link | RMBase: m6A_site_374951 | ||
| mod ID: M6ASITE034532 | Click to Show/Hide the Full List | ||
| mod site | chr17:59930398-59930399:+ | [5] | |
| Sequence | CACCAGTTGTTCCTAACAGAACCATTCTCATTGCGGCTTTC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000472940.5; ENST00000406116.7; ENST00000443572.6; ENST00000489824.1; ENST00000393021.7; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_374952 | ||
| mod ID: M6ASITE034533 | Click to Show/Hide the Full List | ||
| mod site | chr17:59934179-59934180:+ | [7] | |
| Sequence | TTCATTGTAGGTCATGTGAAACTAACAGACTTTGGACTATG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000587622.1; ENST00000472940.5; ENST00000443572.6; ENST00000225577.9; ENST00000590928.1; ENST00000393021.7; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374953 | ||
| mod ID: M6ASITE034534 | Click to Show/Hide the Full List | ||
| mod site | chr17:59934194-59934195:+ | [5] | |
| Sequence | GTGAAACTAACAGACTTTGGACTATGCAAAGAATCTATTCA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000587622.1; ENST00000225577.9; ENST00000406116.7; ENST00000443572.6; ENST00000393021.7; ENST00000590928.1; ENST00000472940.5 | ||
| External Link | RMBase: m6A_site_374954 | ||
| mod ID: M6ASITE034535 | Click to Show/Hide the Full List | ||
| mod site | chr17:59934222-59934223:+ | [5] | |
| Sequence | AAGAATCTATTCATGATGGAACAGTCACACACACATTTTGT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000443572.6; ENST00000393021.7; ENST00000472940.5; ENST00000587622.1; ENST00000590928.1; ENST00000406116.7; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_374955 | ||
| mod ID: M6ASITE034536 | Click to Show/Hide the Full List | ||
| mod site | chr17:59934246-59934247:+ | [5] | |
| Sequence | TCACACACACATTTTGTGGAACAATAGAATACATGTGAGCT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000393021.7; ENST00000587622.1; ENST00000406116.7; ENST00000472940.5; ENST00000443572.6; ENST00000590928.1; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_374956 | ||
| mod ID: M6ASITE034537 | Click to Show/Hide the Full List | ||
| mod site | chr17:59935248-59935249:+ | [5] | |
| Sequence | GACAAAATCCTCAAATGTAAACTCAATTTGCCTCCCTACCT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; DART-seq | ||
| Transcript ID List | ENST00000443572.6; ENST00000225577.9; ENST00000472940.5; ENST00000393021.7; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374957 | ||
| mod ID: M6ASITE034538 | Click to Show/Hide the Full List | ||
| mod site | chr17:59945431-59945432:+ | [7] | |
| Sequence | ACATATGTGGCTCCATCTGTACTTGAAAGTGTGAAAGAAAA | ||
| Motif Score | 3.278136905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000475155.1; ENST00000472940.5; ENST00000225577.9; ENST00000393021.7; ENST00000406116.7; ENST00000443572.6; ENST00000591035.1 | ||
| External Link | RMBase: m6A_site_374958 | ||
| mod ID: M6ASITE034539 | Click to Show/Hide the Full List | ||
| mod site | chr17:59945464-59945465:+ | [5] | |
| Sequence | AAAGAAAAGTTTTCCTTTGAACCAAAAATCCGATCACCTCG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000472940.5; ENST00000475155.1; ENST00000225577.9; ENST00000393021.7; ENST00000406116.7; ENST00000591035.1; ENST00000443572.6 | ||
| External Link | RMBase: m6A_site_374959 | ||
| mod ID: M6ASITE034540 | Click to Show/Hide the Full List | ||
| mod site | chr17:59945507-59945508:+ | [5] | |
| Sequence | GATTTATTGGCAGCCCACGAACACCTGTCAGGTATTTCACA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000472940.5; ENST00000225577.9; ENST00000443572.6; ENST00000591035.1; ENST00000406116.7; ENST00000393021.7; ENST00000475155.1 | ||
| External Link | RMBase: m6A_site_374960 | ||
| mod ID: M6ASITE034541 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946617-59946618:+ | [5] | |
| Sequence | CCAGCACAGCAAATCCTCAGACACCTGTGGAATACCCAATG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; HepG2; U2OS; A549; GM12878; MM6; Huh7; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406116.7; ENST00000591035.1; ENST00000393021.7; ENST00000475155.1; ENST00000472940.5; ENST00000225577.9; ENST00000443572.6 | ||
| External Link | RMBase: m6A_site_374961 | ||
| mod ID: M6ASITE034542 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946641-59946642:+ | [5] | |
| Sequence | CTGTGGAATACCCAATGGAAACAAGTGGCATAGAGCAGATG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; U2OS; hNPCs; hESCs; fibroblasts; A549; GM12878; MM6; Huh7; peripheral-blood; HEK293A-TOA; iSLK; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000475155.1; ENST00000393021.7; ENST00000406116.7; ENST00000591035.1; ENST00000225577.9; ENST00000443572.6; ENST00000472940.5 | ||
| External Link | RMBase: m6A_site_374962 | ||
| mod ID: M6ASITE034543 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946703-59946704:+ | [7] | |
| Sequence | GCATCGGCACCACTTCCAATACGACAGCCGAACTCTGGGCC | ||
| Motif Score | 2.084416667 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406116.7; ENST00000393021.7; ENST00000472940.5; ENST00000591035.1; ENST00000443572.6; ENST00000475155.1; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_374963 | ||
| mod ID: M6ASITE034544 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946706-59946707:+ | [8] | |
| Sequence | TCGGCACCACTTCCAATACGACAGCCGAACTCTGGGCCATA | ||
| Motif Score | 2.865571429 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000393021.7; ENST00000443572.6; ENST00000406116.7; ENST00000225577.9; ENST00000472940.5; ENST00000591035.1; ENST00000475155.1 | ||
| External Link | RMBase: m6A_site_374964 | ||
| mod ID: M6ASITE034545 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946714-59946715:+ | [5] | |
| Sequence | ACTTCCAATACGACAGCCGAACTCTGGGCCATACAAAAAAC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; hNPCs; hESCs; fibroblasts; GM12878; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; TREX; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000475155.1; ENST00000443572.6; ENST00000406116.7; ENST00000225577.9; ENST00000393021.7; ENST00000472940.5; ENST00000591035.1 | ||
| External Link | RMBase: m6A_site_374965 | ||
| mod ID: M6ASITE034546 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946733-59946734:+ | [5] | |
| Sequence | AACTCTGGGCCATACAAAAAACAAGCTTTTCCCATGATCTC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; hNPCs; hESCs; fibroblasts; GM12878; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; TREX; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000472940.5; ENST00000225577.9; ENST00000591035.1; ENST00000475155.1; ENST00000443572.6; ENST00000393021.7; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374966 | ||
| mod ID: M6ASITE034547 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946864-59946865:+ | [8] | |
| Sequence | GCATCCTGCAAGGTGAAACGACTCAAAATGACAGTTTCAGA | ||
| Motif Score | 3.287565476 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000225577.9; ENST00000443572.6; ENST00000472940.5; ENST00000591035.1; ENST00000406116.7; ENST00000475155.1; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_374967 | ||
| mod ID: M6ASITE034548 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946874-59946875:+ | [8] | |
| Sequence | AGGTGAAACGACTCAAAATGACAGTTTCAGAGAGTCAATGT | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | CD8T; A549; AML | ||
| Seq Type List | m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000443572.6; ENST00000225577.9; ENST00000475155.1; ENST00000393021.7; ENST00000472940.5; ENST00000406116.7; ENST00000591035.1 | ||
| External Link | RMBase: m6A_site_374968 | ||
| mod ID: M6ASITE034549 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946906-59946907:+ | [5] | |
| Sequence | AGTCAATGTCATTACATAGAACACTTCAGACACAGGAAAAA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; HEC-1-A; GSCs; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000591035.1; ENST00000443572.6; ENST00000475155.1; ENST00000225577.9; ENST00000406116.7; ENST00000472940.5; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_374969 | ||
| mod ID: M6ASITE034550 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946915-59946916:+ | [5] | |
| Sequence | CATTACATAGAACACTTCAGACACAGGAAAAATAAACGTGG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; DART-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000443572.6; ENST00000472940.5; ENST00000406116.7; ENST00000591035.1; ENST00000393021.7; ENST00000475155.1 | ||
| External Link | RMBase: m6A_site_374970 | ||
| mod ID: M6ASITE034551 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946930-59946931:+ | [7] | |
| Sequence | TTCAGACACAGGAAAAATAAACGTGGATTTTAAAAAATCAA | ||
| Motif Score | 2.179660714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000591035.1; ENST00000406116.7; ENST00000443572.6; ENST00000472940.5; ENST00000475155.1; ENST00000225577.9; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_374971 | ||
| mod ID: M6ASITE034552 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946969-59946970:+ | [5] | |
| Sequence | AATCAATGGTGCAAAAAAAAACTTAAAGCAAAATAGTATTG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000406116.7; ENST00000443572.6; ENST00000225577.9; ENST00000393021.7; ENST00000472940.5; ENST00000591035.1 | ||
| External Link | RMBase: m6A_site_374972 | ||
| mod ID: M6ASITE034553 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946994-59946995:+ | [5] | |
| Sequence | AAGCAAAATAGTATTGCTGAACTCTTAGGCACATCAATTAA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; U2OS; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; MM6; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000393021.7; ENST00000406116.7; ENST00000591035.1; ENST00000472940.5; ENST00000443572.6; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_374973 | ||
| mod ID: M6ASITE034554 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947028-59947029:+ | [7] | |
| Sequence | CAATTAATTGATTCCTCGCGACATCTTCTCAACCTTATCAA | ||
| Motif Score | 2.865571429 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000406116.7; ENST00000591035.1; ENST00000393021.7; ENST00000443572.6; ENST00000225577.9; ENST00000472940.5 | ||
| External Link | RMBase: m6A_site_374974 | ||
| mod ID: M6ASITE034555 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947073-59947074:+ | [5] | |
| Sequence | TTTCATGTTGATGACTCGAAACTGACAGTATTAAGGGTAGG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; hNPCs; hESCs; fibroblasts; GM12878; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000443572.6; ENST00000591035.1; ENST00000225577.9; ENST00000472940.5; ENST00000393021.7; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374975 | ||
| mod ID: M6ASITE034556 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947077-59947078:+ | [9] | |
| Sequence | ATGTTGATGACTCGAAACTGACAGTATTAAGGGTAGGATGT | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | brain; HEK293; kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000591035.1; ENST00000443572.6; ENST00000406116.7; ENST00000472940.5; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_374976 | ||
| mod ID: M6ASITE034557 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947110-59947111:+ | [7] | |
| Sequence | TAGGATGTTGCTTCTGAATCACTGTTGAGTTCTGATTGTGT | ||
| Motif Score | 2.469291667 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000472940.5; ENST00000393021.7; ENST00000406116.7; ENST00000225577.9; ENST00000591035.1; ENST00000443572.6 | ||
| External Link | RMBase: m6A_site_374977 | ||
| mod ID: M6ASITE034558 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947163-59947164:+ | [7] | |
| Sequence | ATCCTTTCATTAGGCAAAGTACAAAATTGCCTATAATACTT | ||
| Motif Score | 2.856142857 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000393021.7; ENST00000472940.5; ENST00000591035.1; ENST00000225577.9; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374978 | ||
| mod ID: M6ASITE034559 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947194-59947195:+ | [5] | |
| Sequence | TATAATACTTGCAACTAAGGACAAATTAGCATGCAAGCTTG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; U2OS; hNPCs; hESCs; fibroblasts; A549; GM12878; Huh7; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406116.7; ENST00000393021.7; ENST00000472940.5; ENST00000225577.9; ENST00000591035.1 | ||
| External Link | RMBase: m6A_site_374979 | ||
| mod ID: M6ASITE034560 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947220-59947221:+ | [5] | |
| Sequence | TAGCATGCAAGCTTGGTCAAACTTTTTCCAGCAAAATGGAA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; hNPCs; hESCs; fibroblasts; A549; GM12878; CD8T; Huh7; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq; DART-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000472940.5; ENST00000393021.7; ENST00000591035.1; ENST00000225577.9; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374980 | ||
| mod ID: M6ASITE034561 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947247-59947248:+ | [5] | |
| Sequence | CCAGCAAAATGGAAGCAAAGACAAAAGAAACTTACCAATTG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; hNPCs; hESCs; fibroblasts; A549; GM12878; Huh7; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq; DART-seq | ||
| Transcript ID List | ENST00000591035.1; ENST00000406116.7; ENST00000225577.9; ENST00000393021.7; ENST00000472940.5 | ||
| External Link | RMBase: m6A_site_374981 | ||
| mod ID: M6ASITE034562 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947256-59947257:+ | [5] | |
| Sequence | TGGAAGCAAAGACAAAAGAAACTTACCAATTGATGTTTTAC | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; hNPCs; hESCs; fibroblasts; A549; GM12878; CD8T; Huh7; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq; DART-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000472940.5; ENST00000406116.7; ENST00000591035.1; ENST00000225577.9; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_374982 | ||
| mod ID: M6ASITE034563 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947260-59947261:+ | [7] | |
| Sequence | AGCAAAGACAAAAGAAACTTACCAATTGATGTTTTACGTGC | ||
| Motif Score | 2.052208333 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000472940.5; ENST00000406116.7; ENST00000393021.7; ENST00000591035.1 | ||
| External Link | RMBase: m6A_site_374983 | ||
| mod ID: M6ASITE034564 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947283-59947284:+ | [5] | |
| Sequence | AATTGATGTTTTACGTGCAAACAACCTGAATCTTTTTTTTA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; hNPCs; hESCs; fibroblasts; A549; GM12878; Huh7; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq; DART-seq | ||
| Transcript ID List | ENST00000406116.7; ENST00000591035.1; ENST00000393021.7; ENST00000225577.9; ENST00000472940.5 | ||
| External Link | RMBase: m6A_site_374984 | ||
| mod ID: M6ASITE034565 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947354-59947355:+ | [5] | |
| Sequence | GATTCAGCTCATTATGAAAAACATCCCAAACTTTAAAATGC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEK293T; HepG2; A549; GM12878; Huh7; HEK293A-TOA | ||
| Seq Type List | m6A-seq; MeRIP-seq; DART-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000393021.7; ENST00000472940.5; ENST00000591035.1; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374985 | ||
| mod ID: M6ASITE034566 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947363-59947364:+ | [5] | |
| Sequence | CATTATGAAAAACATCCCAAACTTTAAAATGCGAAATTATT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HEK293T; HepG2; A549; GM12878; Huh7; HEK293A-TOA | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000406116.7; ENST00000472940.5; ENST00000591035.1; ENST00000225577.9; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_374986 | ||
| mod ID: M6ASITE034567 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947405-59947406:+ | [10] | |
| Sequence | GTTGGTGTGAAGAAAGCCAGACAACTTCTGTTTCTTCTCTT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HEK293T; HepG2; GM12878; Huh7 | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000591035.1; ENST00000472940.5; ENST00000393021.7; ENST00000225577.9; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374987 | ||
| mod ID: M6ASITE034568 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947408-59947409:+ | [7] | |
| Sequence | GGTGTGAAGAAAGCCAGACAACTTCTGTTTCTTCTCTTGGT | ||
| Motif Score | 2.595904762 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000472940.5; ENST00000406116.7; ENST00000591035.1; ENST00000393021.7; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_374988 | ||
| mod ID: M6ASITE034569 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947495-59947496:+ | [10] | |
| Sequence | CGTTTGAGGGATTGGGGTGGACCTGGGGTTTATTTTCAGTA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HEK293T; HepG2; Huh7 | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000406116.7; ENST00000591035.1; ENST00000225577.9; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_374989 | ||
| mod ID: M6ASITE034570 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947675-59947676:+ | [7] | |
| Sequence | TTGTCCCGGGCCTGCATTGCACTGGAAAAAAAAATCGCCAC | ||
| Motif Score | 3.252583333 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000591035.1; ENST00000225577.9; ENST00000393021.7; ENST00000587061.1; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374990 | ||
| mod ID: M6ASITE034571 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947724-59947725:+ | ||
| Sequence | ACACCAGTATTTGGTTCAAGACACCAAATGTCTTCAGCCCA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000591035.1; ENST00000406116.7; ENST00000225577.9; ENST00000587061.1; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_374991 | ||
| mod ID: M6ASITE034572 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947755-59947756:+ | ||
| Sequence | CTTCAGCCCATGGCTGAAGAACAACAGAAGAGAGTCAGGAT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000406116.7; ENST00000393021.7; ENST00000591035.1; ENST00000587061.1; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_374992 | ||
| mod ID: M6ASITE034573 | Click to Show/Hide the Full List | ||
| mod site | chr17:59947947-59947948:+ | [7] | |
| Sequence | TCCTTATATTTTTTTCCTCAACAGTTTTAAAAAGAAAAAAA | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000587061.1; ENST00000225577.9; ENST00000591035.1; ENST00000406116.7; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_374993 | ||
| mod ID: M6ASITE034574 | Click to Show/Hide the Full List | ||
| mod site | chr17:59948049-59948050:+ | [9] | |
| Sequence | CCTTTTGATGAATGTCTTCCACAGTAAAGAAAACTTAGTGG | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | HEK293; HEK293T | ||
| Seq Type List | m6A-REF-seq; DART-seq | ||
| Transcript ID List | ENST00000587061.1; ENST00000225577.9; ENST00000591035.1; ENST00000406116.7; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_374994 | ||
| mod ID: M6ASITE034575 | Click to Show/Hide the Full List | ||
| mod site | chr17:59948061-59948062:+ | [7] | |
| Sequence | TGTCTTCCACAGTAAAGAAAACTTAGTGGCTTAATTTAGGA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000591035.1; ENST00000393021.7; ENST00000225577.9; ENST00000587061.1; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374995 | ||
| mod ID: M6ASITE034576 | Click to Show/Hide the Full List | ||
| mod site | chr17:59948118-59948119:+ | [7] | |
| Sequence | ACTATGTTTTTGAAATTGTAACAAAATCTACATAAATGATT | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000587061.1; ENST00000393021.7; ENST00000591035.1; ENST00000406116.7; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_374996 | ||
| mod ID: M6ASITE034577 | Click to Show/Hide the Full List | ||
| mod site | chr17:59948168-59948169:+ | [7] | |
| Sequence | AAAGAATAAAAATAAAGGTAACTTTACCTTTCTTAAATATT | ||
| Motif Score | 2.590089286 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000393021.7; ENST00000591035.1; ENST00000225577.9; ENST00000587061.1; ENST00000406116.7 | ||
| External Link | RMBase: m6A_site_374997 | ||
| mod ID: M6ASITE034578 | Click to Show/Hide the Full List | ||
| mod site | chr17:59948216-59948217:+ | [7] | |
| Sequence | TTAAAGAGAGCATTTCCATGACTTTAGCTGGTGAAAGGGTT | ||
| Motif Score | 3.28175 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000591035.1; ENST00000225577.9; ENST00000393021.7; ENST00000587061.1 | ||
| External Link | RMBase: m6A_site_374998 | ||
| mod ID: M6ASITE034579 | Click to Show/Hide the Full List | ||
| mod site | chr17:59948647-59948648:+ | [10] | |
| Sequence | CGATAAAAGTTCATCTTTGGACAGAAAGCCTTTAAAAAAAA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HEK293T; HeLa | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000591035.1; ENST00000587061.1; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_374999 | ||
| mod ID: M6ASITE034580 | Click to Show/Hide the Full List | ||
| mod site | chr17:59948716-59948717:+ | [5] | |
| Sequence | ATTGCAGCAGCCTACTGAGAACTTTGACTGTTGCTGGTAAA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000591035.1; ENST00000587061.1; ENST00000225577.9; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_375000 | ||
| mod ID: M6ASITE034581 | Click to Show/Hide the Full List | ||
| mod site | chr17:59948746-59948747:+ | [7] | |
| Sequence | TTGCTGGTAAATTAGAAGCTACAATAATAATTAAGGGCAGA | ||
| Motif Score | 2.078666667 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000591035.1; ENST00000587061.1; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_375001 | ||
| mod ID: M6ASITE034582 | Click to Show/Hide the Full List | ||
| mod site | chr17:59948773-59948774:+ | [7] | |
| Sequence | TAATTAAGGGCAGAAATTATACTTAAAAAGTGCAGATCCTT | ||
| Motif Score | 2.53247619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000587061.1; ENST00000393021.7; ENST00000591035.1; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_375002 | ||
| mod ID: M6ASITE034583 | Click to Show/Hide the Full List | ||
| mod site | chr17:59949042-59949043:+ | [5] | |
| Sequence | GCTATAACCACCCCAGTTAAACCATTTTCATAATTAGTAGT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; DART-seq | ||
| Transcript ID List | ENST00000591035.1; ENST00000225577.9; ENST00000393021.7; ENST00000587061.1 | ||
| External Link | RMBase: m6A_site_375003 | ||
| mod ID: M6ASITE034584 | Click to Show/Hide the Full List | ||
| mod site | chr17:59949206-59949207:+ | [5] | |
| Sequence | CTGTGGAAAGTTTATTGAGAACTTGTTTCATAAATGGATAT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000587061.1; ENST00000393021.7; ENST00000591035.1; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_375004 | ||
| mod ID: M6ASITE034585 | Click to Show/Hide the Full List | ||
| mod site | chr17:59949246-59949247:+ | [5] | |
| Sequence | TCCCTACTATGACTGTGAAAACATGTCAAGTGTCACATTAG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; DART-seq | ||
| Transcript ID List | ENST00000587061.1; ENST00000591035.1; ENST00000225577.9; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_375005 | ||
| mod ID: M6ASITE034586 | Click to Show/Hide the Full List | ||
| mod site | chr17:59949275-59949276:+ | [5] | |
| Sequence | GTGTCACATTAGTGTCACAGACAGAAAGCACACACCTATGC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000591035.1; ENST00000225577.9; ENST00000587061.1; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_375006 | ||
| mod ID: M6ASITE034587 | Click to Show/Hide the Full List | ||
| mod site | chr17:59949360-59949361:+ | [7] | |
| Sequence | TAAAATATGATGTCGATATTACTAGTCTTGAGTTTCTAAGA | ||
| Motif Score | 2.494845238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000587061.1; ENST00000393021.7; ENST00000591035.1; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_375007 | ||
| mod ID: M6ASITE034588 | Click to Show/Hide the Full List | ||
| mod site | chr17:59949521-59949522:+ | [7] | |
| Sequence | TTTTTAATTAAAATATAATCACTGAAGTTTACTAATTTGAT | ||
| Motif Score | 2.469291667 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000587061.1; ENST00000393021.7; ENST00000591035.1 | ||
| External Link | RMBase: m6A_site_375008 | ||
| mod ID: M6ASITE034589 | Click to Show/Hide the Full List | ||
| mod site | chr17:59949531-59949532:+ | [7] | |
| Sequence | AAATATAATCACTGAAGTTTACTAATTTGATTTTATAAGGT | ||
| Motif Score | 2.494845238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000591035.1; ENST00000225577.9; ENST00000393021.7; ENST00000587061.1 | ||
| External Link | RMBase: m6A_site_375009 | ||
| mod ID: M6ASITE034590 | Click to Show/Hide the Full List | ||
| mod site | chr17:59949682-59949683:+ | [7] | |
| Sequence | TTTCCCCCTTTATGGTCTTAACTAATTTGAATCCTTCAAGA | ||
| Motif Score | 2.590089286 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000393021.7; ENST00000591035.1; ENST00000587061.1; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_375010 | ||
| mod ID: M6ASITE034591 | Click to Show/Hide the Full List | ||
| mod site | chr17:59949787-59949788:+ | [7] | |
| Sequence | TATGATACTCCATAAATTCAACATTCTTTACTATAGGTAAT | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000591035.1; ENST00000587061.1; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_375011 | ||
| mod ID: M6ASITE034592 | Click to Show/Hide the Full List | ||
| mod site | chr17:59949820-59949821:+ | [7] | |
| Sequence | TAGGTAATGAATGATTATAAACAAGATGCATCTTAGATAGT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000587061.1; ENST00000393021.7; ENST00000591035.1 | ||
| External Link | RMBase: m6A_site_375012 | ||
| mod ID: M6ASITE034593 | Click to Show/Hide the Full List | ||
| mod site | chr17:59949849-59949850:+ | [7] | |
| Sequence | ATCTTAGATAGTATTAATATACTGAGCCTTGGATTATATAT | ||
| Motif Score | 2.53247619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000587061.1; ENST00000225577.9; ENST00000591035.1; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_375013 | ||
| mod ID: M6ASITE034594 | Click to Show/Hide the Full List | ||
| mod site | chr17:59949880-59949881:+ | [7] | |
| Sequence | GATTATATATTTAATATAGGACCTATTTTGAATATTCAGTT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000591035.1; ENST00000393021.7; ENST00000587061.1; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_375014 | ||
| mod ID: M6ASITE034595 | Click to Show/Hide the Full List | ||
| mod site | chr17:59949921-59949922:+ | [7] | |
| Sequence | AATCATATGGTTCCTAGCTTACAAGGGCTAGATCTAAGATT | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000393021.7; ENST00000225577.9; ENST00000587061.1; ENST00000591035.1 | ||
| External Link | RMBase: m6A_site_375015 | ||
| mod ID: M6ASITE034596 | Click to Show/Hide the Full List | ||
| mod site | chr17:59950067-59950068:+ | [7] | |
| Sequence | AGTAGACACTAGCAAGCTGGACAAACTATCACAAAAGTATT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000225577.9; ENST00000587061.1; ENST00000591035.1; ENST00000393021.7 | ||
| External Link | RMBase: m6A_site_375016 | ||
| mod ID: M6ASITE034597 | Click to Show/Hide the Full List | ||
| mod site | chr17:59950150-59950151:+ | [7] | |
| Sequence | TTTTTCTTGTGTGATTCTTAACATTATAGCACAAGTATTAT | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000393021.7; ENST00000587061.1; ENST00000225577.9; ENST00000591035.1 | ||
| External Link | RMBase: m6A_site_375017 | ||
| mod ID: M6ASITE034598 | Click to Show/Hide the Full List | ||
| mod site | chr17:59950236-59950237:+ | [5] | |
| Sequence | TGTTTGTGTTTGCTCTTTAAACTATTGTTTCTCCTATCCCA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000587061.1; ENST00000393021.7; ENST00000591035.1; ENST00000225577.9 | ||
| External Link | RMBase: m6A_site_375018 | ||
Pseudouridine (Pseudo)
| In total 1 m6A sequence/site(s) in this target gene | |||
| mod ID: PSESITE000108 | Click to Show/Hide the Full List | ||
| mod site | chr17:59946741-59946742:+ | [11] | |
| Sequence | GCCATACAAAAAACAAGCTTTTCCCATGATCTCCAAACGGC | ||
| Transcript ID List | ENST00000443572.6; ENST00000393021.7; ENST00000406116.7; ENST00000225577.9; ENST00000472940.5; ENST00000475155.1; ENST00000591035.1 | ||
| External Link | RMBase: Pseudo_site_2252 | ||
References