m6A Regulator Information
General Information of the m6A Regulator (ID: REG00018)
| Regulator Name | Methyltransferase-like 5 (METTL5) | ||||
|---|---|---|---|---|---|
| Synonyms |
rRNA N6-adenosine-methyltransferase METTL5; DC3; HSPC133
Click to Show/Hide
|
||||
| Gene Name | METTL5 | ||||
| Sequence |
MKKVRLKELESRLQQVDGFEKPKLLLEQYPTRPHIAACMLYTIHNTYDDIENKVVADLGC
GCGVLSIGTAMLGAGLCVGFDIDEDALEIFNRNAEEFELTNIDMVQCDVCLLSNRMSKSF DTVIMNPPFGTKNNKGTDMAFLKTALEMARTAVYSLHKSSTREHVQKKAAEWKIKIDIIA ELRYDLPASYKFHKKKSVDIEVDLIRFSF Click to Show/Hide
|
||||
| Family | methyltransferase superfamily; PrmA family | ||||
| Function |
Catalytic subunit of a heterodimer with TRMT112, which specifically methylates the 6th position of adenine in position 1832 of 18S rRNA. N6-methylation of adenine(1832) in 18S rRNA resides in the decoding center of 18S rRNA and is required for translation and embryonic stem cells (ESCs) pluripotency and differentiation.
Click to Show/Hide
|
||||
| Gene ID | 29081 | ||||
| Uniprot ID | |||||
| Regulator Type | WRITER ERASER READER | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
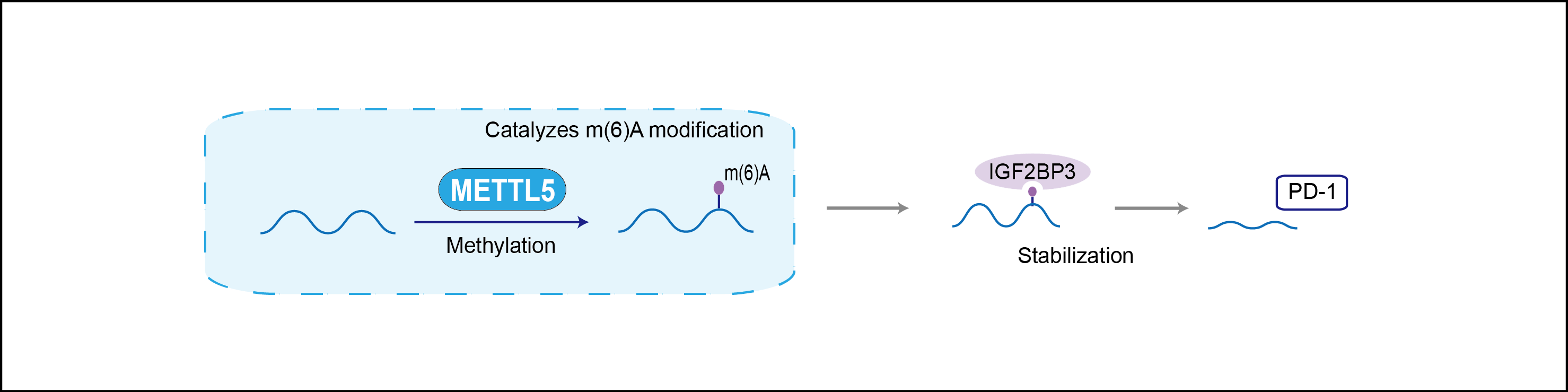
|
|||||
Full List of Target Gene(s) of This m6A Regulator and Corresponding Disease/Drug Response(s)
METTL5 can regulate the m6A methylation of following target genes, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulted from the regulation of certain target gene.
Browse Target Gene related Disease
Myc proto-oncogene protein (MYC)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [1] | |||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| Cell Process | Cell proliferation | |||
| Cell migration | ||||
| Cell invasion | ||||
| Response Summary | The study revealed important roles for METTL5 in the development of pancreatic cancer and present the METTL5/Myc proto-oncogene protein (MYC) axis as a novel therapeutic strategy for treatment. | |||
DNA mismatch repair protein Msh2 (MSH2)
Endometrial cancer [ICD-11: 2C76]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [2] | |||
| Responsed Disease | Endometrial cancer [ICD-11: 2C76] | |||
| Target Regulation | Up regulation | |||
| Pathway Response | Mismatch repair | hsa03430 | ||
| Cell Process | DNA mismatch repair | |||
| Microsatellite instability | ||||
In-vitro Model |
CP-H058 (Normal endometrial cells) | |||
| KLE | Endometrial adenocarcinoma | Homo sapiens | CVCL_1329 | |
| RL95-2 | Endometrial adenosquamous carcinoma | Homo sapiens | CVCL_0505 | |
| Ishikawa | Endometrial adenocarcinoma | Homo sapiens | CVCL_2529 | |
| ECC-1 | Endometrial Cancer | Homo sapiens | CVCL_7260 | |
| In-vivo Model | The male BALB/c nude mice were randomized divide into two groups, each group including six 4 weeks old nude mice. Investigators were blinded to the treatment groups during data collection and subsequent data analysis. In the subcutaneous xenograft model, 5 × 105 cells were subcutaneously injected in the right flanks of nude mice. In the orthotopic intracranial mouse model, each mouse was intracranially injected with 1 × 105 luciferase transfected U87MG cells in 10 uL PBS solution. | |||
| Response Summary | Knocking down METTL5 could significantly activate apoptosis and inhibit endometrial carcinoma(EC) development via MMR administration.METTL5 expression in UCEC tumor tissue was increased, and UCEC patients with high METTL5 expression had worse prognostic outcomes. METTL5 knockdown induced the DNA mismatch repair protein Msh2 (MSH2), MSH6 and PMS2 expression in MMR. | |||
DNA mismatch repair protein Msh6 (MSH6)
Endometrial cancer [ICD-11: 2C76]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [2] | |||
| Responsed Disease | Endometrial cancer [ICD-11: 2C76] | |||
| Target Regulation | Up regulation | |||
| Pathway Response | Mismatch repair | hsa03430 | ||
| Cell Process | DNA mismatch repair | |||
| Microsatellite instability | ||||
In-vitro Model |
CP-H058 (Normal endometrial cells) | |||
| KLE | Endometrial adenocarcinoma | Homo sapiens | CVCL_1329 | |
| RL95-2 | Endometrial adenosquamous carcinoma | Homo sapiens | CVCL_0505 | |
| Ishikawa | Endometrial adenocarcinoma | Homo sapiens | CVCL_2529 | |
| ECC-1 | Endometrial Cancer | Homo sapiens | CVCL_7260 | |
| In-vivo Model | The male BALB/c nude mice were randomized divide into two groups, each group including six 4 weeks old nude mice. Investigators were blinded to the treatment groups during data collection and subsequent data analysis. In the subcutaneous xenograft model, 5 × 105 cells were subcutaneously injected in the right flanks of nude mice. In the orthotopic intracranial mouse model, each mouse was intracranially injected with 1 × 105 luciferase transfected U87MG cells in 10 uL PBS solution. | |||
| Response Summary | Knocking down METTL5 could significantly activate apoptosis and inhibit endometrial carcinoma(EC) development via MMR administration.METTL5 expression in UCEC tumor tissue was increased, and UCEC patients with high METTL5 expression had worse prognostic outcomes. METTL5 knockdown induced the MSH2, DNA mismatch repair protein Msh6 (MSH6) and PMS2 expression in MMR. | |||
Mismatch repair endonuclease PMS2 (PMS2)
Endometrial cancer [ICD-11: 2C76]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [2] | |||
| Responsed Disease | Endometrial cancer [ICD-11: 2C76] | |||
| Target Regulation | Up regulation | |||
| Pathway Response | Mismatch repair | hsa03430 | ||
| Cell Process | DNA mismatch repair | |||
| Microsatellite instability | ||||
In-vitro Model |
CP-H058 (Normal endometrial cells) | |||
| KLE | Endometrial adenocarcinoma | Homo sapiens | CVCL_1329 | |
| RL95-2 | Endometrial adenosquamous carcinoma | Homo sapiens | CVCL_0505 | |
| Ishikawa | Endometrial adenocarcinoma | Homo sapiens | CVCL_2529 | |
| ECC-1 | Endometrial Cancer | Homo sapiens | CVCL_7260 | |
| In-vivo Model | The male BALB/c nude mice were randomized divide into two groups, each group including six 4 weeks old nude mice. Investigators were blinded to the treatment groups during data collection and subsequent data analysis. In the subcutaneous xenograft model, 5 × 105 cells were subcutaneously injected in the right flanks of nude mice. In the orthotopic intracranial mouse model, each mouse was intracranially injected with 1 × 105 luciferase transfected U87MG cells in 10 uL PBS solution. | |||
| Response Summary | Knocking down METTL5 could significantly activate apoptosis and inhibit endometrial carcinoma(EC) development via MMR administration.METTL5 expression in UCEC tumor tissue was increased, and UCEC patients with high METTL5 expression had worse prognostic outcomes. METTL5 knockdown induced the MSH2, MSH6 and Mismatch repair endonuclease PMS2 (PMS2) expression in MMR. | |||
18S rRNA
Liver cancer [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [3] | |||
| Responsed Disease | Intrahepatic cholangiocarcinoma [ICD-11: 2C12.10] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
HEK293T | Normal | Homo sapiens | CVCL_0063 |
| In-vivo Model | A total of 1 × 106 HuCCT1-sgNC and HuCCT1-sgMETTL5 cells in 0.2 mL of PBS were separately injected into 6-week-old male NCG mice (N = 7 per group) via caudal vein. Mice were sacrificed at 4 weeks after injection, and the lung tissues were processed into 4-mm-thick paraffin-embedded sections. H&E staining was subsequently performed to determine the pulmonary metastasis. | |||
Heat shock factor protein 4 (HSF4B)
Nasopharyngeal carcinoma [ICD-11: 2B6B]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [4] | |||
| Responsed Disease | Nasopharyngeal carcinoma [ICD-11: 2B6B] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
C666-1 | Nasopharyngeal carcinoma | Homo sapiens | CVCL_7949 |
| HK1 | Nasopharyngeal carcinoma | Acipenser baerii | CVCL_YE27 | |
| HONE-1 | Nasopharyngeal carcinoma | Homo sapiens | CVCL_8706 | |
| S18 | Nasopharyngeal carcinoma | Homo sapiens | CVCL_B0U9 | |
| S26 | Nasopharyngeal carcinoma | Homo sapiens | CVCL_B0UB | |
| 5-8F | Nasopharyngeal carcinoma | Homo sapiens | CVCL_C528 | |
| 6-10B | Nasopharyngeal carcinoma | Homo sapiens | CVCL_C529 | |
| HNE-1 | Nasopharyngeal carcinoma | Homo sapiens | CVCL_0308 | |
| In-vivo Model | Female BALB/c nude mice aged 5 weeks were used to perform in vivo proliferation and metastasis assays. For subcutaneous xenograft tumor model, 1×106 6-10B cells in 200 μL PBS were injected into the dorsal flank of each mouse (n = 7 in each group). The long and short diameters of subcutaneous tumors were measured every 3 days until the endpoint. Mice were sacrificed about 4 weeks after injection, and subcutaneous tumors were harvested, weighted and then fixed in 4% paraformaldehyde for subsequent analysis. For inguinal lymph node metastasis model, 1×105 5-8F cells in 30 μL of PBS were injected into the right foot pad of each mouse (n = 7 in each group). Mice were sacrificed about one month after injection. Primary foot pad tumor and metastatic inguinal lymph nodes were harvested and fixed in 4% paraformaldehyde for subsequent assays. | |||
Unspecific Target Gene
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [5] | |||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | |||
| Pathway Response | Nucleotide excision repair | hsa03420), Oxidative phosphorylation | ||
| Cell Process | Oxidative phosphorylation | |||
| Nucleotide excision repair | ||||
| Mismatch repair | ||||
| Response Summary | METTL5 protein was decreased in GCTs compared with AIMTs and ANTs, and it is a potential prognostic biomarker in GC. | |||
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [6] | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
In-vitro Model |
HEK293T | Normal | Homo sapiens | CVCL_0063 |
| HeLa | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| MCF7/LCC9 | Invasive breast carcinoma | Homo sapiens | CVCL_DP52 | |
| MDA-MB-231 | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| T-47D | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | |
| ZR75-30 | Breast cancer | Homo sapiens | CVCL_1661 | |
| Response Summary | METTL5 expression is elevated in breast cancer patient samples and is required for growth of several breast cancer cell lines. | |||
Intellectual disability [ICD-11: 6A00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [7] | |||
| Responsed Disease | Intellectual disability [ICD-11: 6A00] | |||
In-vitro Model |
HEK293T | Normal | Homo sapiens | CVCL_0063 |
| In-vivo Model | Mettl5 +/- mice with C57BL/6N background were generated by using CRISPR-Cas9 systems. The exon 2, exon 3 and exon 4 of Mettl5 were deleted in the knockout Mettl5 allele. Mice were genotyped for the targeted allele by PCR using tail DNA. | |||
| Response Summary | Mettl5 knockout in mESCs leads to the abnormal craniofacial and nervous development. Moreover, using Mettl5 knockout mouse model, we further demonstrated that Mettl5 knockout mice exhibit intellectual disability, recapitulating the human phenotype. | |||
Full List of Crosstalk(s) between m6A Modification and Epigenetic Regulation Related to This Regulator
Non-coding RNA
m6A Target: 18S rRNA
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05695 | ||
| Epigenetic Regulator | 18S rRNA | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Intrahepatic cholangiocarcinoma | |
References