m6A-centered Drug Response Information
General Information of the Drug (ID: M6APDG02941)
| Name |
HONOKIOL
|
||||
|---|---|---|---|---|---|
| Synonyms |
Honokiol; 35354-74-6; 5,3'-Diallyl-2,4'-dihydroxybiphenyl; NSC 293100; Honokiol,(S); UNII-11513CCO0N; 3,5'-Diallyl-4,2'-dihydroxybiphenyl; 3',5-Diallylbiphenyl-2,4'-diol; CPD000387107; CHEMBL16901; CHEBI:5759; 4-allyl-2-(3-allyl-4-hydroxy-phenyl)phenol; 3',5-diallyl-[1,1'-biphenyl]-2,4'-diol; 11513CCO0N; 3',5-diallyl-2,4'-biphenyldiol; AK-25837; 5,3& -Diallyl-2,4& Q-100425; [1,1'-Biphenyl]-2,4'-diol, 3',5-di-2-propenyl-; SMR000387107; 3',5-di(prop-2-en-1-yl)biphenyl-2,4'-diol; houpa
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Structure |
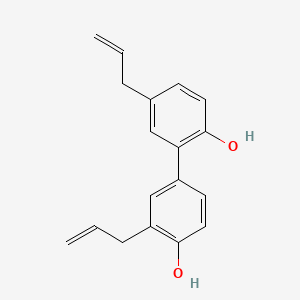 |
||||
| Formula |
C18H18O2
|
||||
| InChI |
1S/C18H18O2/c1-3-5-13-7-9-18(20)16(11-13)14-8-10-17(19)15(12-14)6-4-2/h3-4,7-12,19-20H,1-2,5-6H2
|
||||
| InChIKey |
FVYXIJYOAGAUQK-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Target Gene(s) and Their Upstream m6A Regulator, Together with the Effect of Target Gene(s) in Drug Response
The target genes involved in drug-target interaction (such as drug-metabolizing enzymes, drug transporters and therapeutic targets) and drug-mediated cell death signaling (including modulating DNA damage and repair capacity, escaping from drug-induced apoptosis, autophagy, cellular metabolic reprogramming, oncogenic bypass signaling, cell microenvironment, cell stemness, etc.) could be regulated by m6A regulator(s) and affected their corresponding drug response. You can browse detailed information on drug-related target gene(s) mediated by m6A regulators.
Prostaglandin G/H synthase 2 (COX-2)
Methyltransferase-like 3 (METTL3)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | Prostaglandin G/H synthase 2 (COX-2) is a therapeutic target for HONOKIOL. The Methyltransferase-like 3 (METTL3) has potential in affecting the response of HONOKIOL through regulating the expression of Prostaglandin G/H synthase 2 (COX-2). | [1], [2] | ||
References