m6A-centered Drug Response Information
General Information of the Drug (ID: M6ADRUG0102)
| Name |
Bortezomib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Bortezomib; 179324-69-7; Velcade; PS-341; Bortezomib (PS-341); LDP-341; Ps 341; ((R)-3-Methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxamido)propanamido)butyl)boronic acid; DPBA; LDP 341; UNII-69G8BD63PP; [(1R)-3-methyl-1-[[(2S)-3-phenyl-2-(pyrazine-2-carbonylamino)propanoyl]amino]butyl]boronic acid; Bortezomib (Velcade); N-[(1R)-1-(DIHYDROXYBORYL)-3-METHYLBUTYL]-N-(PYRAZIN-2-YLCARBONYL)-L-PHENYLALANINAMIDE; MLN-341; [(1R)-3-Methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl)amino]propyl]amino]butyl]boronic acid; MG 341; MG-341; Boronic acid, B-[(1R)-3-methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(2-pyrazinylcarbonyl)amino]propyl]amino]butyl]-; NSC 681239; NSC-681239; 69G8BD63PP; CHEMBL325041; Peptide boronate; CHEBI:52717; MFCD09056737; NSC681239; NCGC00242506-02; S1013; DSSTox_CID_20980; DSSTox_RID_79609; N-[(1R)-1-(dihydroxyboranyl)-3-methylbutyl]-Nalpha-(pyrazin-2-ylcarbonyl)-L-phenylalaninamide; DSSTox_GSID_40980; radiciol; Pyz-Phe-boroLeu; (R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxamido)propanamido)butylboronic acid; [(1R)-3-methyl-1-({(2S)-3-phenyl-2-[(pyrazin-2-ylcarbonyl)amino]propanoyl}amino)butyl]boronic acid; Velcade (TN); CAS-179324-69-7; PS 341 (pharmaceutical); Brotezamide; PROSCRIPT BORONIC ACID; Bortezomib [USAN:INN:BAN]; Bortezomib accord; C19H25BN4O4; HSDB 7666; Bortezomib hydrate; Bortezomib,Velcade; LPD 341; LPD-341; Velcade, Radiciol; NCGC00168751-01; NCGC00181022-01; Bortezomib- Bio-X; Velcade (Millenium); 3mg0; MLS004774142; Bortezomib (JAN/USAN/INN); SCHEMBL192129; GTPL6391; DTXSID3040980; AOB6368; ((1R)-3-Methyl-1-(((2S)-3-phenyl-2-((pyrazinylcarbonyl)amino)propanoyl)amino)butyl)boronic acid; Tox21_112630; Tox21_112672; BDBM50069989; NSC756655; AKOS015909706; Tox21_112672_1; ZINC169746649; AM81235; CCG-268449; CS-1039; DB00188; NSC-756655; Bortezomib (Velcade,MG-341,PS-341); NCGC00242506-01; NCGC00242506-06; NCGC00242506-07; AS-15721; BB164258; HY-10227; NCI60_029010; Q425; SMR003500787; SW208077-3; A18332; D03150; AB01273951-01; AB01273951-02; AB01273951_03; 324B697; Q419319; SR-01000939863; SR-01000939863-2; BRD-K88510285-001-02-0; (R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-6-carboxamido)propanamido)butylboronic acid; (1R)-3-Methyl-1-({(2S)-3-phenyl-2-[(2-pyrazinylcarbonyl)amino]propanoyl}amino)butylboronic acid, AldrichCPR; (R)-3-METHYL-1-((S)-3-PHENYL-2-(PYRAZINE-2-CARBOXAMIDO)PROPANAMIDO)BUTAN-2-YLBORONIC ACID; [(1R)-3-Methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl)amino]propyl]amino]-butyl]boronic acid; Boronic acid, ((1R)-3-methyl-1-(((2S)-1-oxo-3-phenyl-2-((pyrazinylcarbonyl)amino)propyl)amino)butyl)-; Boronic acid, (3-methyl-1-((1-oxo-3-phenyl-2-((pyrazinylcarbonyl)amino)propyl)amino)butyl)-, (S-(R*,S*))-; Boronic acid, [(1(R)-3-methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl)amino]propyl]amino]butyl]-; N-((1S)-1-Benzyl-2-(((1R)-1-(dihydroxyboranyl)-3-methylbutyl)amino)-2-oxoethyl)pyrazinecarboxamide; N-((1S)-1-benzyl-2-(((1R)-1-(dihydroxyboranyl)-3-methylbutyl)amino)2-oxoethylpyrazinecarboxamide; N-[(1R)-1-(dihydroxyboranyl)-3-methylbutyl]-N(alpha)-(pyrazin-2-ylcarbonyl)-L-phenylalaninamide
Click to Show/Hide
|
||||
| Status | Approved | [1] | |||
| Structure |
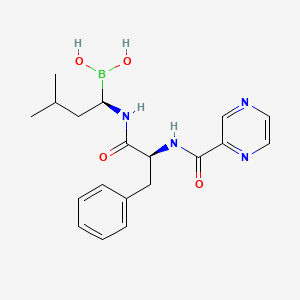 |
||||
|
3D MOL
|
|||||
| Formula |
C19H25BN4O4
|
||||
| InChI |
InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1
|
||||
| InChIKey |
GXJABQQUPOEUTA-RDJZCZTQSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Full List of m6A Targets Related to This Drug
Heat shock factor protein 1 (HSF1)
| In total 1 item(s) under this target gene | ||||
| Experiment 1 Reporting the m6A-centered Drug Response by This Target Gene | [2] | |||
| Response Summary | FTO significantly promotes MM cell proliferation, migration, and invasion by targeting Heat shock factor protein 1 (HSF1)/HSPs in a YTHDF2-dependent manner. FTO inhibition, especially when combined with bortezomib (BTZ) treatment, synergistically inhibited myeloma bone tumor formation and extramedullary spread in NCG mice. | |||
| Responsed Disease | Multiple myeloma | ICD-11: 2A83.1 | ||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Up regulation | |||
| In-vitro Model | RPMI-8226 | Plasma cell myeloma | Homo sapiens | CVCL_0014 |
| MM1.R | Plasma cell myeloma | Homo sapiens | CVCL_8794 | |
| In-vivo Model | A total of 3×106 RPMI8226/MM1R-Luc cells were intravenously injected into NCG mice to establish a disseminated human MM xenograft model. The in vivo antitumor effect of the FTO inhibitor MA2 combined with or without the first-line chemotherapeutic agent BTZ was evaluated as follows: 3 days post xenotransplantation, MA2 (20 mg/kg), or vehicle control was injected intraperitoneally (i.p.) daily for 10 days, and BTZ was injected intraperitoneally on days 1, 4, 8, and 11. Mouse serum was collected at specified time points during the treatment, and the tumor burden was monitored by detecting myeloma cell-secreted Lambda light chains via a Human Lambda ELISA Kit (Bethyl Laboratories, No. E88-116). Tumor development was monitored weekly after treatment with an in vivo imaging system (IVIS, SI Imaging, Lago, and LagoX). Luciferin (150 mg/kg, YEASEN, Shanghai, China) was injected intraperitoneally into the mice. | |||
Superoxide dismutase [Mn], mitochondrial (SOD2)
| In total 1 item(s) under this target gene | ||||
| Experiment 1 Reporting the m6A-centered Drug Response by This Target Gene | [3] | |||
| Response Summary | FTO promotes Bortezomib resistance via m6A-dependent destabilization of Superoxide dismutase [Mn], mitochondrial (SOD2) expression in multiple myeloma. | |||
| Responsed Disease | Multiple myeloma | ICD-11: 2A83.1 | ||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Down regulation | |||
References