m6A-centered Drug Response Information
General Information of the Drug (ID: M6ADRUG0094)
| Name |
RG7112
|
||||
|---|---|---|---|---|---|
| Synonyms |
RG7112; 939981-39-2; RG-7112; RO5045337; RO-5045337; UNII-Q8MI0X869M; RG 7112; Q8MI0X869M; CHEMBL2386346; ((4S,5R)-2-(4-(tert-Butyl)-2-ethoxyphenyl)-4,5-bis(4-chlorophenyl)-4,5-dimethyl-4,5-dihydro-1H-imidazol-1-yl)(4-(3-(methylsulfonyl)propyl)piperazin-1-yl)methanone; [(4S,5R)-2-(4-tert-butyl-2-ethoxyphenyl)-4,5-bis(4-chlorophenyl)-4,5-dimethylimidazol-1-yl]-[4-(3-methylsulfonylpropyl)piperazin-1-yl]methanone; Ro 5045337; GTPL9599; Mdm2 antagonist ro5045337; SCHEMBL12704861; DTXSID60240182; BCP07937; EX-A1686; BDBM50434287; NSC809100; s7030; ZINC96270381; CCG-270414; CS-0330; DB14793; NSC-809100; SB19057; AC-32970; HY-10959; RO5045337; RG7112; RG7112 (RO5045337); R 7112; A857519; Q27287118; RG-7112;RG 7112; RO-5045337; RO 5045337; RO5045337; [(4R,5S)-4,5-Bis(4-chlorophenyl)-2-[4-(1,1-dimethylethyl)-2-ethoxyphenyl]-4,5-dihydro-4,5-dimethyl-1H-imidazol-1-yl][4-[3-(methylsulfonyl)propyl]-1-piperazinyl]methanone;R7112 RG7112 RG 7112; RG7112; RO5045337; RO 5045337; RO5045337; [(4s,5r)-2-(4-t-butyl-2-ethoxyphenyl)-4,5-bis(4-chlorophenyl)-4,5-dimethylimidazol-1-yl]-[4-(3-methylsulfonylpropyl)piperazin-1-yl]methanone; Methanone, [(4S,5R)-4,5-bis(4-chlorophenyl)-2-[4-(1,1-dimethylethyl)-2-ethoxyphenyl]-4,5-dihydro-4,5-dimethyl-1H-imidazol-1-yl][4-[3-(methylsulfonyl)propyl]-1-piperazinyl]-
Click to Show/Hide
|
||||
| Status | Phase 1 | [1] | |||
| Structure |
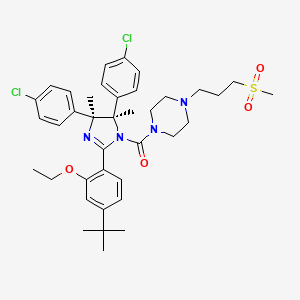 |
||||
| Formula |
C38H48Cl2N4O4S
|
||||
| InChI |
InChI=1S/C38H48Cl2N4O4S/c1-8-48-33-26-29(36(2,3)4)14-19-32(33)34-41-37(5,27-10-15-30(39)16-11-27)38(6,28-12-17-31(40)18-13-28)44(34)35(45)43-23-21-42(22-24-43)20-9-25-49(7,46)47/h10-19,26H,8-9,20-25H2,1-7H3/t37-,38+/m0/s1
|
||||
| InChIKey |
QBGKPEROWUKSBK-QPPIDDCLSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of m6A Targets Related to This Drug
Cellular tumor antigen p53 (TP53/p53)
| In total 1 item(s) under this target gene | ||||
| Experiment 1 Reporting the m6A-centered Drug Response by This Target Gene | [2] | |||
| Response Summary | Cellular tumor antigen p53 (TP53/p53) n6-methyladenosine (m6A) played a decisive role in regulating Hepatocellular carcinoma(HCC) sensitivity to chemotherapy via the p53 activator RG7112 and the vascular endothelial growth factor receptor inhibitor apatinib. p53 mRNA m6A modification blockage induced by S-adenosyl homocysteine or siRNA-mediated METTL3 inhibition enhanced HCC sensitivity to chemotherapy. | |||
| Responsed Disease | Hepatocellular carcinoma | ICD-11: 2C12.02 | ||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | p53 signaling pathway | hsa04115 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell apoptosis | |||
| In-vitro Model | QGY-7701 | Human papillomavirus-related endocervical adenocarcinoma | Homo sapiens | CVCL_6859 |
| HHL-5 | Normal | Homo sapiens | CVCL_S956 | |
| Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| In-vivo Model | Nude mice (4-6 week-old) were administered sterile water and feed in a specific pathogen-free barrier. Using a 1-mL syringe, 1 × 107 HEPG2 cells were subcutaneously inoculated into the right axilla of nude mice to build the HCC xenograft model. When the tumor volume reached 50 mm3, the nude mice were randomly divided into 1 control (n = 4) and 3 treatment groups (n = 4 each). RG7112, apatinib, and RG7112 + apatinib were administered to the treatment groups and an equal volume of dimethyl sulfoxide to the control group by daily gavage for 14 d. The tumor length (L) and width (W) were measured on alternate days using vernier calipers. The following formula was used to calculate the tumor volume: volume (mm3) = 0.5 × L × W × W. At the end of the experiment, the nude mice were killed by CO2 overdose anesthesia. The tumors were dissected and weighed using a precision balance, and the tumor tissue was stored in liquid nitrogen for further analysis. | |||
References