m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05975
|
[1] | |||
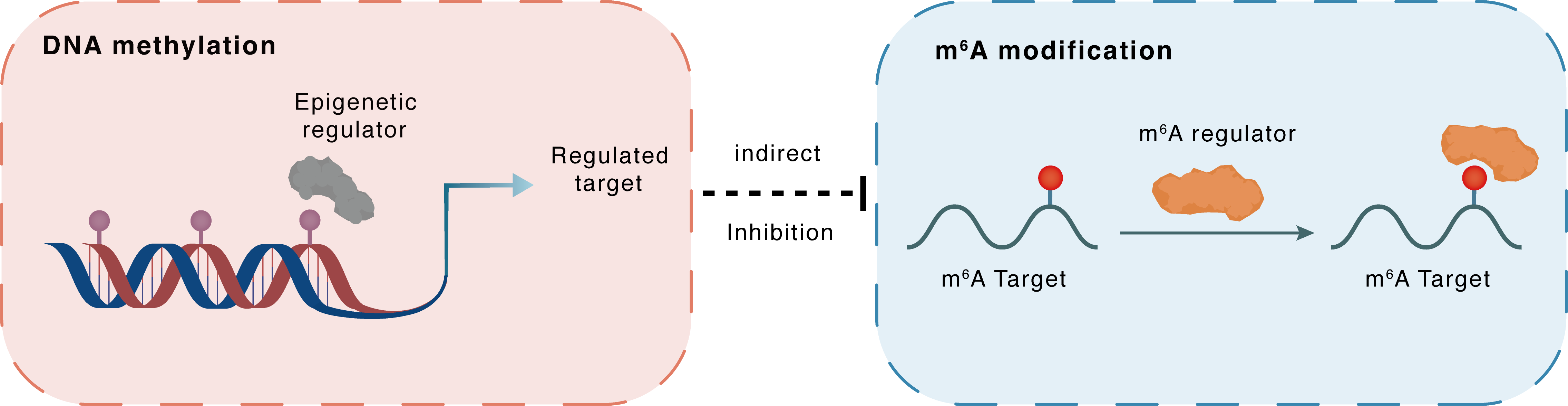 DNA methylation
MeCP2
HOXD10
Indirect
Inhibition
m6A modification
m6A Target
m6A Target
IGFBP3
DNA methylation
MeCP2
HOXD10
Indirect
Inhibition
m6A modification
m6A Target
m6A Target
IGFBP3
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Insulin-like growth factor-binding protein 3 (IGFBP3) | READER | |||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Epigenetic Regulator | Methyl-CpG-binding protein 2 (MECP2) | WRITER | View Details | ||
| Regulated Target | Homeobox D10 (HOXD10) | View Details | |||
| Crosstalk Relationship | DNA methylation → m6A | Inhibition | |||
| Crosstalk Mechanism | DNA methylation indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | Functionally, Homeobox D10 (HOXD10) acts as a tumor suppressor gene, in which HOXD10-expressing cells showed suppressed cell proliferation, colony formation ability, and migration and invasion capacity. Mechanistically, DNMT1, DNMT3B, and MeCP2 were recruited in the HOXD10 promoter, and demethylation by 5-Aza-2'-deoxycytidine (5-Aza-CdR) treatment or MeCP2 knockdown can sufficiently induce HOXD10 expression. HOXD10regulates the expressions of miR-7 and IGFBP3 in a promoter-dependent manner. | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Responsed Drug | 5-FU | ||||
| Cell Process | Cell proliferation | ||||
| Cell colony formation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In-vitro Model |
HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Insulin-like growth factor-binding protein 3 (IGFBP3) | 1 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Salvianolic acid B | Phase 2 | [2] | ||
| Synonyms |
115939-25-8; 121521-90-2; Salvianolic-acid-B; Lithospermic acid B; MFCD07779133; (2R)-2-[(E)-3-[(2R,3R)-3-[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy]carbonyl-2-(3,4-dihydroxyphenyl)-7-hydroxy-2,3-dihydro-1-benzofuran-4-yl]prop-2-enoyl]oxy-3-(3,4-dihydroxyphenyl)propanoic acid; (R)-2-(((2R,3R)-4-((E)-3-((R)-1-Carboxy-2-(3,4-dihydroxyphenyl)ethoxy)-3-oxoprop-1-en-1-yl)-2-(3,4-dihydroxyphenyl)-7-hydroxy-2,3-dihydrobenzofuran-3-carbonyl)oxy)-3-(3,4-dihydroxyphenyl)propanoic acid; Danshensuan B; Salcianolic acid B; C36H30O16; PubChem13035; CHEMBL3747259; ZINC49538629; AKOS015920258; AS-56980; S609; 939S258; (R)-2-((2R,3R)-4-((E)-3-((R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy)-3-oxoprop-1-enyl)-2-(3,4-dihydroxyphenyl)-7-hydroxy-2,3-dihydrobenzofuran-3-carbonyloxy)-3-(3,4-dihydroxyphenyl)propanoic acid; 1607436-77-0
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Methyl-CpG-binding protein 2 (MECP2) | 1 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| AVXS-201 | Phase 1 | [3] | ||
| External Link | ||||
| 2B91: Colorectal cancer | 25 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Retifanlimab | Approved | [4] | ||
| Synonyms |
INCMGA0012; Retifanlimab
Click to Show/Hide
|
|||
| External Link | ||||
| Aflibercept | Approved | [5] | ||
| Synonyms |
Ziv-Aflibercept; Zaltrap (TN); VEGF Trap; VEGF Trap-Eye
Click to Show/Hide
|
|||
| External Link | ||||
| Regorafenib | Approved | [6] | ||
| Synonyms |
755037-03-7; BAY 73-4506; Regorafenibum; Stivarga; 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; BAY73-4506; Regorafenib (BAY 73-4506); UNII-24T2A1DOYB; 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide; BAY-73-4506; 24T2A1DOYB; CHEMBL1946170; CHEBI:68647; Stivarga (TN); BAY73-4506 hydrochloride; Regorafenib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [7] | ||
| Synonyms |
Bevacizumab (ophthalmic slow-release tissue tablet)
Click to Show/Hide
|
|||
| External Link | ||||
| SYM-004 | Phase 3 | [7] | ||
| Synonyms |
Chimeric IgG1 antibody 1024 (cancer), Symphogen; Chimeric IgG1 antibody 992 (cancer), Symphogen; Chimeric IgG1 antibodies992 + 1024 (cancer), Symphogen
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab + Erlotinib | Phase 3 | [8] | ||
| External Link | ||||
| CPI-613 | Phase 3 | [7] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [5] | ||
| External Link | ||||
| AlloStim | Phase 2/3 | [9] | ||
| Synonyms |
AlloStim (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Sibrotuzumab | Phase 2 | [10] | ||
| External Link | ||||
| CV301 | Phase 2 | [11] | ||
| External Link | ||||
| Efatutazone | Phase 2 | [12] | ||
| Synonyms |
Inolitazone; 223132-37-4; 5-[[4-[[6-(4-amino-3,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-yl]methoxy]phenyl]methyl]-2,4-Thiazolidinedione; Efatutazone [INN]; RS5444; CS-7017; SCHEMBL3246054; CHEMBL3545280; JCYNMRJCUYVDBC-UHFFFAOYSA-N; Efatutazone;CS-7017;RS5444; BCP07478; AKOS030526729; DB11894; CS-0778; KB-77905; DA-07988; HY-14792; QC-10456; 4CA-1384; FT-0737589; 5-[4-[6-(4-amino-3 ,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-ylmethoxy]benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| LOR-2040 | Phase 2 | [13] | ||
| External Link | ||||
| RG7221 | Phase 2 | [14] | ||
| External Link | ||||
| PEG-SN38 | Phase 2 | [15] | ||
| Synonyms |
EZN-2208
Click to Show/Hide
|
|||
| External Link | ||||
| MEGF0444A | Phase 2 | [16] | ||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [17] | ||
| External Link | ||||
| AB928 | Phase 1/2 | [18] | ||
| External Link | ||||
| MGD007 | Phase 1 | [14] | ||
| External Link | ||||
| BNC-101 | Phase 1 | [19] | ||
| External Link | ||||
| Navicixizumab | Phase 1 | [7] | ||
| External Link | ||||
| RG7160 | Discontinued in Phase 2 | [20] | ||
| External Link | ||||
| Nimesulide | Terminated | [21] | ||
| Synonyms |
51803-78-2; N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide; Mesulid; Flogovital; Sulidene; Nimed; R-805; 4-NITRO-2-PHENOXYMETHANESULFONANILIDE; Nisulid; Nimesulidum [INN-Latin]; Nimesulida [INN-Spanish]; R 805; UNII-V4TKW1454M; 4-Nitro-2-phenoxy-methanesulfonanilide; 4'-Nitro-2'-phenoxymethanesulfonanilide; Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-; EINECS 257-431-4; 4'-Nitro-2'-phenoxymethansulfonanilid; BRN 2421175; CHEMBL56367; MLS000069680; V4TKW1454M; Methanesulfonanilide, 4'-nitro-2'-phenoxy-; CHEBI:44445; Dulanermin
Click to Show/Hide
|
|||
| External Link | ||||
| Saracatinib | Phase 2 | [22] | ||
| External Link | ||||
| G3139 + Irinotecan | Investigative | [23] | ||
| External Link | ||||
References