m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05836
|
[1] | |||
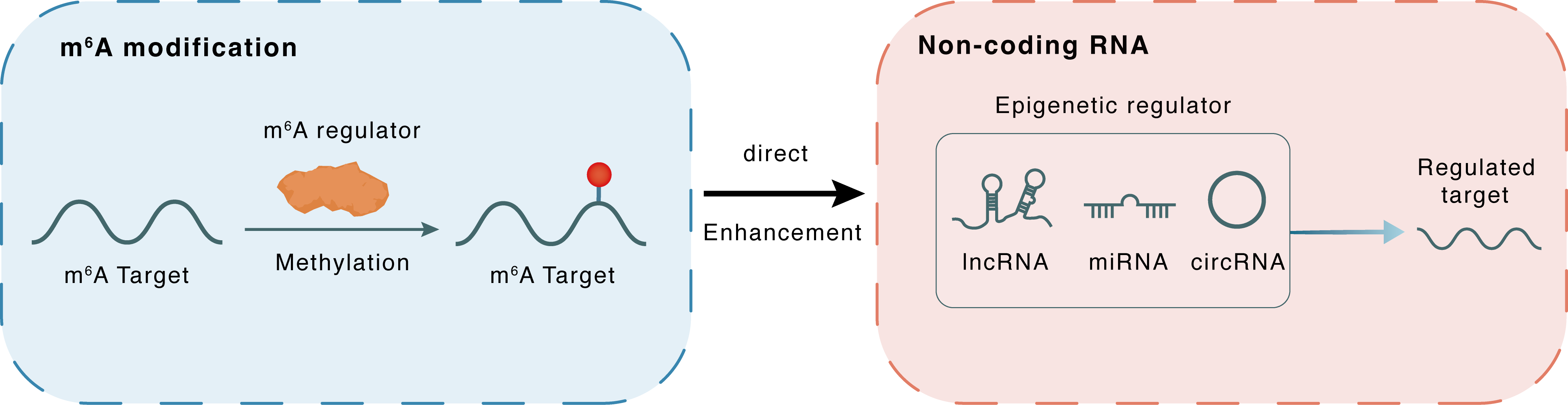 m6A modification
XIST
XIST
METTL14
Methylation
m6A modification
XIST
XIST
METTL14
Methylation
 : m6A sites
Direct
Inhibition
Non-coding RNA
XIST
Regulated Target
lncRNA miRNA circRNA : m6A sites
Direct
Inhibition
Non-coding RNA
XIST
Regulated Target
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 14 (METTL14) | WRITER | |||
| m6A Target | X inactive specific transcript (XIST) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | X inactive specific transcript (XIST) | LncRNA | View Details | ||
| Crosstalk Relationship | m6A → ncRNA | Inhibition | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | Because methylation regulates X inactive specific transcript (XIST) expression, the differences in m6A-related genes expressed between the high and low XIST expression groups were analyzed. The expression of METTL14 in the low XIST expression group was notably increased compared with that in the high XIST expression group (P = 0.03276), which indicated that METTL14 participated in XIST m6A-methylation. | ||||
| Responsed Disease | Diffuse large B-cell lymphomas | ICD-11: 2A81 | |||
| Cell Process | Cell proliferation | ||||
In-vitro Model |
OCI-Ly3 | Diffuse large B-cell lymphoma activated B-cell type | Homo sapiens | CVCL_8800 | |
| OCI-Ly10 | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_8795 | ||
| SU-DHL-2 | Diffuse large B-cell lymphoma activated B-cell type | Homo sapiens | CVCL_9550 | ||
| U-2932 | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1896 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2A81: Diffuse large B-cell lymphomas | 93 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Glofitamab | Approved | [2] | ||
| Synonyms |
Glofitamab
Click to Show/Hide
|
|||
| External Link | ||||
| Epcoritamab | Approved | [3] | ||
| External Link | ||||
| Loncastuximab tesirine | Approved | [4] | ||
| External Link | ||||
| Pozelimab | Approved | [5] | ||
| Synonyms |
REGN3918
Click to Show/Hide
|
|||
| External Link | ||||
| Ocrelizumab | Approved | [6] | ||
| Synonyms |
Ocrelizumab (USAN); Ocrelizumab (genetical recombination); Ocrelizumab (genetical recombination) (JAN)
Click to Show/Hide
|
|||
| External Link | ||||
| Ofatumumab | Phase 3 | [7] | ||
| Synonyms |
Arzerra (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Tafasitamab | Approved | [8] | ||
| Synonyms |
Xmab 5574/MOR208
Click to Show/Hide
|
|||
| External Link | ||||
| Umbralisib | Phase 2/3 | [9] | ||
| Synonyms |
RP5264; TGR-1202
Click to Show/Hide
|
|||
| External Link | ||||
| Lisocabtagene maraleucel | Approved | [10] | ||
| Synonyms |
JCAR017
Click to Show/Hide
|
|||
| External Link | ||||
| Durvalumab | Approved | [11] | ||
| External Link | ||||
| Blinatumomab | Phase 2/3 | [11] | ||
| Synonyms |
AMG 103; Blincyto
Click to Show/Hide
|
|||
| External Link | ||||
| Tisagenlecleucel | Application submitted | [11] | ||
| Synonyms |
Tisagenlecleucel-T
Click to Show/Hide
|
|||
| External Link | ||||
| Axicabtagene ciloleucel | Approved | [6] | ||
| External Link | ||||
| Ibrutinib | Phase 3 | [11] | ||
| Synonyms |
PCI-32765; Ibrutinib (BTK inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Pembrolizumab | Approved | [11] | ||
| External Link | ||||
| Polatuzumab vedotin | Approved | [12] | ||
| External Link | ||||
| Nivolumab | Approved | [11] | ||
| External Link | ||||
| Tazemetostat | Approved | [11] | ||
| Synonyms |
EPZ-6438; 1403254-99-8; EPZ6438; UNII-Q40W93WPE1; N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide; Q40W93WPE1; EPZ 6438; E7438; (1,1'-Biphenyl)-3-carboxamide, N-((1,2-dihydro-4,6-dimethyl-2-oxo-3-pyridinyl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(4-morpholinylmethyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| ICP-022 | Phase 2 | [13] | ||
| Synonyms |
Orelabrutinib; 1655504-04-3; UNII-WJA5UO9E10; WJA5UO9E10; 4-yl]pyridine-3-carboxamide; ICP022; 2-(4-phenoxyphenyl)-6-[1-(prop-2-enoyl)piperidin-; 6-(1-acryloylpiperidin-4-yl)-2-(4-phenoxyphenyl)nicotinamide; Orelabrutinib [INN]; orelabrutinib (proposed INN); SCHEMBL16597834; US9951056, Example 3; GTPL10629; BDBM389631; EX-A3442; NSC826039; s9600; NSC-826039; example 3 [WO2015048662A2]; DB-091042; HY-129390; CS-0105163; 2-(4-phenoxyphenyl)-6-(1-prop-2-enoylpiperidin-4-yl)pyridine-3-carboxamide; 3-Pyridinecarboxamide, 6-(1-(1-oxo-2-propen-1-yl)-4-piperidinyl)-2-(4-phenoxyphenyl)-; 6-(1-(1-Oxo-2-propen-1-yl)-4-piperidinyl)-2-(4-phenoxyphenyl)-3-pyridinecarboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| BI-695500 | Application submitted | [11] | ||
| Synonyms |
Rituximab biosimilar
Click to Show/Hide
|
|||
| External Link | ||||
| Abexinostat | Phase 3 | [11] | ||
| Synonyms |
PCI-24781; 783355-60-2; PCI 24781; CRA-024781; CRA 024781; UNII-IYO470654U; 3-((dimethylamino)methyl)-N-(2-(4-(hydroxycarbamoyl)phenoxy)ethyl)benzofuran-2-carboxamide; CRA-02478; Abexinostat(PCI-24781); PCI-24781 (Abexinostat); Abexinostat (PCI-24781); IYO470654U; 3-[(Dimethylamino)methyl]-N-[2-[4-[(hydroxyamino)carbonyl]phenoxy]ethyl]-2-benzofurancarboxamide; 3-((Dimethylamino)methyl)-N-(2-(4-(hydroxycarbamoyl)-phenoxy)ethyl)benzofuran-2-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| Pixantrone | Phase 3 | [14] | ||
| Synonyms |
Pixuvri (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Enzastaurin | Phase 3 | [11] | ||
| Synonyms |
LY317615; LE-0014; LY317615, Enzastaurin; 3-(1-methyl-1H-indol-3-yl)-4-{1-[1-(pyridin-2-ylmethyl)piperidin-4-yl]-1H-indol-3-yl}-1H-pyrrole-2,5-dione; 3-(1-methylindol-3-yl)-4-[1-[1-(pyridin-2-ylmethyl)piperidin-4-yl]indol-3-yl]pyrrole-2,5-dione
Click to Show/Hide
|
|||
| External Link | ||||
| Adcetris | Phase 3 | [15] | ||
| External Link | ||||
| CC-486 | Phase 3 | [11] | ||
| Synonyms |
AG-14361; AG14361; 328543-09-5; UNII-48N0U0K50I; AG 14361; CHEMBL65892; 48N0U0K50I; Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-; AG-014361; 1-(4-((dimethylamino)methyl)phenyl)-8,9-dihydro-2,7,9a-triazabenzo[cd]azulen-6(7H)-one; Imidazo(4,5,1-jk)(1,4)benzodiazepin-7(4H)-one, 2-(4-((dimethylamino)methyl)phenyl)-5,6-dihydro-; 2-[4-[(Dimethylamino)methyl]phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one; SMR000486393; MLS006011157; MLS001065917; Nucleoside analogue
Click to Show/Hide
|
|||
| External Link | ||||
| GP2013 | Phase 3 | [15] | ||
| External Link | ||||
| Telcyta canfosfamide | Phase 3 | [16] | ||
| External Link | ||||
| VR876 | Phase 3 | [17] | ||
| External Link | ||||
| Selinexor | Phase 3 | [18] | ||
| Synonyms |
Xpovio; KPT 330; KPT-330; KPT-330(Selinexor); KPT330;Selinexor; 1393477-72-9; 31TZ62FO8F; CHEMBL3545185; SCHEMBL14678327; Selinexor (KPT-330); Selinexor [USAN:INN]; Tube706; UNII-31TZ62FO8F
Click to Show/Hide
|
|||
| External Link | ||||
| Zilovertamab vedotin | Phase 2/3 | [19] | ||
| Synonyms |
MK-2140
Click to Show/Hide
|
|||
| External Link | ||||
| MOR-208 | Phase 2/3 | [18] | ||
| Synonyms |
XmAb CD19; XENP-5574; XENP-5603; XmAb-5574; Anti-CD19 humanized mAb (cancer/autoimmune disease), Xencor; Anti-CD19 humanized monoclonal antibody (cancer/autoimmune disease), Xencor
Click to Show/Hide
|
|||
| External Link | ||||
| MT-3724 | Phase 2 | [20] | ||
| External Link | ||||
| Plamotamab | Phase 2 | [21] | ||
| Synonyms |
XmAb 13676
Click to Show/Hide
|
|||
| External Link | ||||
| PF-07901801 | Phase 2 | [22] | ||
| Synonyms |
maplirpacept
Click to Show/Hide
|
|||
| External Link | ||||
| DPX Survivac | Phase 2 | [23] | ||
| External Link | ||||
| Naratuximab emtansine | Phase 2 | [24] | ||
| Synonyms |
DEBIO 1562
Click to Show/Hide
|
|||
| External Link | ||||
| CD20 CAR T cells | Phase 2 | [25] | ||
| External Link | ||||
| Baltaleucel-T | Phase 2 | [15] | ||
| External Link | ||||
| SGN-CD19A | Phase 2 | [26] | ||
| Synonyms |
Denintuzumab mafodotin
Click to Show/Hide
|
|||
| External Link | ||||
| DCDS-4501A | Phase 2 | [27] | ||
| External Link | ||||
| Cobomarsen | Phase 1 | [11] | ||
| Synonyms |
MRG-106
Click to Show/Hide
|
|||
| External Link | ||||
| CD19 CAR T cells | Phase 2 | [25] | ||
| External Link | ||||
| IMO-8400 | Phase 2 | [18] | ||
| Synonyms |
Bazlitoran; Bazlitoran [INN]; Bazlitoran [USAN]; UNII-2U46M95B5M; 2U46M95B5M
Click to Show/Hide
|
|||
| External Link | ||||
| CTL019 | Phase 2 | [28] | ||
| External Link | ||||
| CART-19 | Phase 1 | [29] | ||
| External Link | ||||
| BI-836826 | Phase 2 | [11] | ||
| External Link | ||||
| Pidilizumab | Phase 2 | [30] | ||
| Synonyms |
CT-011
Click to Show/Hide
|
|||
| External Link | ||||
| INCB50465 | Phase 2 | [11] | ||
| External Link | ||||
| PNT-2258 | Phase 2 | [18] | ||
| External Link | ||||
| TAK-659 | Phase 2 | [11] | ||
| External Link | ||||
| DEBIO 1562 | Phase 2 | [11] | ||
| Synonyms |
MGN529
Click to Show/Hide
|
|||
| External Link | ||||
| Denintuzumab mafodotin | Phase 2 | [11] | ||
| External Link | ||||
| Coltuximab ravtansine | Phase 2 | [11] | ||
| External Link | ||||
| MGCD-0103 | Phase 2 | [18] | ||
| Synonyms |
Mocetinostat; MG 0103; MG 4230; MG 4915; MG 5026; MG0103; MG4230; MG4915; MG5206; MGCD 0103; MGCD0103; MG-0103; MG-4230; MG-4915; MG-5026; Mocetinostat, MGCD0103; N-(2-aminophenyl)-4-[[(4-pyridin-3-ylpyrimidin-2-yl)amino]methyl]benzamide; N-(2-Aminophenyl)-4-((4-pyridin-3-ylpyrimidin-2-ylamino)methyl)benzamide
Click to Show/Hide
|
|||
| External Link | ||||
| CX2029 | Phase 1/2 | [31] | ||
| External Link | ||||
| GEN3013 | Phase 1/2 | [32] | ||
| External Link | ||||
| ALLO-501A | Phase 1/2 | [33] | ||
| External Link | ||||
| CD19 CAR T Cells | Phase 1/2 | [34] | ||
| External Link | ||||
| Anti-CD20-CAR vector-transduced autologous T cells | Phase 1/2 | [35] | ||
| External Link | ||||
| MAK683 | Phase 1/2 | [11] | ||
| Synonyms |
XLIBABIFOBYHSV-UHFFFAOYSA-N; EED inhibitor-1; 1951408-58-4; MAK-683; N-[(5-fluoro-2,3-dihydro-1-benzofuran-4-yl)methyl]-8-(2-methylpyridin-3-yl)-[1,2,4]triazolo[4,3-c]pyrimidin-5-amine; N-((5-fluoro-2,3-dihydrobenzofuran-4-yl)methyl)-8-(2-methylpyridin-3-yl)-[1,2,4]triazolo[4,3-c]pyrimidin-5-amine; SCHEMBL17841485; EX-A1723; BCP29116; ACN-053195; CS-8054; AC-30344; HY-103663
Click to Show/Hide
|
|||
| External Link | ||||
| JCAR014 | Phase 1 | [36] | ||
| External Link | ||||
| Anti-CD20 CAR-T cells | Phase 1/2 | [37] | ||
| External Link | ||||
| PCAR-019 | Phase 1/2 | [38] | ||
| External Link | ||||
| Anti-CD19-CAR PBL | Phase 1/2 | [39] | ||
| External Link | ||||
| KTE-C19 CAR | Phase 1/2 | [40] | ||
| External Link | ||||
| SNS01-T | Phase 1/2 | [41] | ||
| External Link | ||||
| CD19.CAR-T cells | Phase 1/2 | [42] | ||
| External Link | ||||
| CUDC-907 | Phase 1 | [43] | ||
| Synonyms |
1339928-25-4; Fimepinostat; CUDC 907; UNII-3S9RX35S5X; CUDC907; 3S9RX35S5X; CHEMBL3622533; N-hydroxy-2-[[2-(6-methoxypyridin-3-yl)-4-morpholin-4-ylthieno[3,2-d]pyrimidin-6-yl]methyl-methylamino]pyrimidine-5-carboxamide; Fimepinostat [USAN]; PI3K/HDAC Inhibitor centn; MLS006010994; SCHEMBL1284705; GTPL8952; KS-00000TDO; EX-A742; AOB6775; DTXSID90712307; MolPort-023-293-550; JOWXJLIFIIOYMS-UHFFFAOYSA-N; HMS3656H04; BCP06870; s2759; BDBM50188961; 2341AH; ZINC73488511; ABP001045; AKOS026750340; SB16569; CUDC-907 (PI3K/HDAC Inhibi
Click to Show/Hide
|
|||
| External Link | ||||
| Betalutin | Phase 1 | [11] | ||
| External Link | ||||
| Anti-CD19/22-CAR vector-transduced T cells | Phase 1/2 | [44] | ||
| External Link | ||||
| JCAR017 | Phase 1 | [45] | ||
| External Link | ||||
| Anti-CD19 CAR-T cells | Phase 1/2 | [46] | ||
| External Link | ||||
| Anti-CD19-CAR vector-transduced T cells | Clinical trial | [47] | ||
| External Link | ||||
| AUTO3 | Phase 1/2 | [48] | ||
| External Link | ||||
| Vmab + Y-90 Emab | Phase 1b | [49] | ||
| External Link | ||||
| Iomab-ACT | Phase 1 | [50] | ||
| External Link | ||||
| CD19t-haNK | Phase 1 | [51] | ||
| External Link | ||||
| KITE-363 | Phase 1 | [52] | ||
| External Link | ||||
| PF-06821497 | Phase 1 | [53] | ||
| Synonyms |
UNII-S4L4MM20B6; S4L4MM20B6; CHEMBL4080228; PF06821497; 1844849-10-0; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1(2H)-one; SCHEMBL17330426; GTPL10516; BDBM50246967; NSC800019; DB14799; HY-101571A; NSC-800019; compound 23a [PMID: 29211475]; CS-0092626; Q29209799; 1(2H)-Isoquinolinone, 5,8-dichloro-2-((1,2-dihydro-4-methoxy-6-methyl-2-oxo-3-pyridinyl)methyl)-3,4-dihydro-7-((R)-methoxy-3-oxetanylmethyl)-; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1-one; CJD
Click to Show/Hide
|
|||
| External Link | ||||
| AMG 562 | Phase 1 | [54] | ||
| External Link | ||||
| Veltuzumab/epratuzumab Y-90 | Phase 1 | [55] | ||
| External Link | ||||
| LIlotomab satetraxetan | Phase 1 | [15] | ||
| External Link | ||||
| Retroviral vector-transduced autologous T cells to express CD22-specific CARs | Phase 1 | [56] | ||
| External Link | ||||
| YTB323 | Phase 1 | [57] | ||
| External Link | ||||
| ASP3026 | Phase 1 | [58] | ||
| Synonyms |
1097917-15-1; ASP-3026; ASP 3026; N2-[2-Methoxy-4-[4-(4-methyl-1-piperazinyl)-1-piperidinyl]phenyl]-N4-[2-[(1-methylethyl)sulfonyl]phenyl]-1,3,5-triazine-2,4-diamine; UNII-HP4L6MXF10; HP4L6MXF10; 2-N-[2-methoxy-4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]phenyl]-4-N-(2-propan-2-ylsulfonylphenyl)-1,3,5-triazine-2,4-diamine; MGGBYMDAPCCKCT-UHFFFAOYSA-N; MLS006011176; GTPL7740; SCHEMBL2827739; CHEMBL3545360; C29H40N8O3S; QCR-144; EX-A140; DTXSID90149038; AOB6601; MolPort-028-720-342; BCP06436; 2229AH; ZINC68120928; s8054
Click to Show/Hide
|
|||
| External Link | ||||
| ME-401 | Phase 1 | [11] | ||
| External Link | ||||
| Human CD19 targeted T Cells | Phase 1 | [59] | ||
| External Link | ||||
| CC-122 | Phase 1 | [11] | ||
| Synonyms |
1015474-32-4; Avadomide; 3-(5-Amino-2-methyl-4-oxoquinazolin-3(4H)-yl)piperidine-2,6-dione; CC122; CC 122; 3-(5-amino-2-methyl-4-oxo-4H-quinazolin-3-yl)-piperidine-2,6-dione; 2,6-Piperidinedione, 3-(5-amino-2-methyl-4-oxo-3(4H)-quinazolinyl)-;2,6-Piperidinedione, 3-(5-amino-2-methyl-4-oxo-3(4H)-quinazolinyl)-; Avadomide [USAN]; Avadomide(CC-122); Avadomide (USAN/INN); SCHEMBL282749; US9694015, Compound A; CHEMBL3989934; BDBM76986; RSNPAKAFCAAMBH-UHFFFAOYSA-N; EX-A1191; BCP15938; s7892; AKOS025399378; SB18829; CS-5995
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-CD19/20-CAR vector-transduced T cells | Clinical trial | [60] | ||
| External Link | ||||
| DCDT-2980S | Discontinued in Phase 2 | [61] | ||
| External Link | ||||
| IM0-8400 | Discontinued in Phase 1/2 | [62] | ||
| External Link | ||||
| Dacetuzumab | Terminated | [63] | ||
| Synonyms |
SMR000449312; MLS000758226; MLS001424020; CHEMBL1408911; CCG-100789
Click to Show/Hide
|
|||
| External Link | ||||
| WHI-P154 | Investigative | [64] | ||
| External Link | ||||
References