m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05466
|
[1] | |||
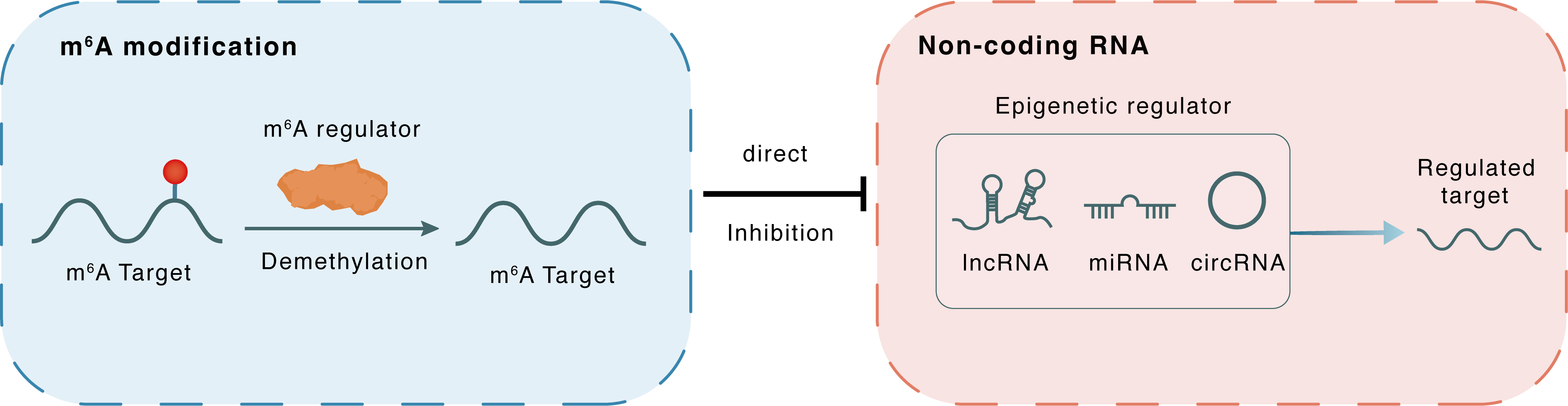 m6A modification
hsa-mir-181b-1
hsa-mir-181b-1
ALKBH5
Demethylation
m6A modification
hsa-mir-181b-1
hsa-mir-181b-1
ALKBH5
Demethylation
 : m6A sites
Direct
Inhibition
Non-coding RNA
miR-181b-1
YAP1
lncRNA miRNA circRNA : m6A sites
Direct
Inhibition
Non-coding RNA
miR-181b-1
YAP1
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | RNA demethylase ALKBH5 (ALKBH5) | ERASER | |||
| m6A Target | hsa-mir-181b-1 | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa-mir-181b-1 | microRNA | View Details | ||
| Regulated Target | Transcriptional coactivator YAP1 (YAP1) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Inhibition | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | ALKBH5 is an anti-tumor factor or a pro-apoptotic factor, acting at least partially by suppressing Transcriptional coactivator YAP1 (YAP1) expression through dual mechanisms with direct m6A methylation of YAP and indirect downregulation of YAP level due to methylation of hsa-mir-181b-1. Further results revealed that m6A methylated pre-miR-181b-1 was subsequently recognized by m6A-binding protein YTHDF2 to mediate RNA degradation. However, methylated YAP transcripts were recognized by YTHDF1 to promote its translation. ALKBH5 overexpression was considered a new approach of replacement therapy for osteosarcoma treatment. | ||||
| Responsed Disease | Osteosarcoma | ICD-11: 2B51 | |||
| Pathway Response | Apoptosis | hsa04210 | |||
| Cell Process | Cell growth | ||||
| Cell migration | |||||
| Cell invasion | |||||
| Cell apoptosis | |||||
In-vitro Model |
U2OS | Osteosarcoma | Homo sapiens | CVCL_0042 | |
| In-vivo Model | Three-week-old BABL/c female nude mice were randomized into three groups. 5 × 106 143B cells were subcutaneously injected in mice, and the tumor volume was assessed every 2 weeks. Eight weeks after injection, the animals were killed. The xenograft tumors were harvested and the tumor volumes were calculated by the standard formula: length × width2/2. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2B51: Osteosarcoma | 12 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Cisplatin | Approved | [2] | ||
| Synonyms |
Abiplatin; Biocisplatinum; Briplatin; Cismaplat; Cisplatine; Cisplatino; Cisplatinum; Cisplatyl; Citoplationo; Lederplatin; Neoplatin; Plastin; Platamine; Platidiam; Platinoxan; Randa; Cis-DDP; Cis-Diamminedichloroplatinum; Peyrone's chloride; Peyrone's salt; Cis-Dichlorodiammineplatinum(II); Cis-[PtCl2(NH3)2]; Cis-diamminedichloridoplatinum(II); Trans-diamminedichloridoplatinum(II); (SP-4-1)-diamminedichloridoplatinum; (SP-4-1)-diamminedichloroplatinum; (SP-4-2)-diamminedichloridoplatinum; (SP-4-2)-diamminedichloroplatinum; Cisplatin (Chemotherapy)
Click to Show/Hide
|
|||
| External Link | ||||
| Naxitamab | Phase 2 | [2] | ||
| External Link | ||||
| Tideglusib | Phase 2 | [3] | ||
| Synonyms |
NP-031112; NP-12; NP031112; Tideglusib(NP-031112)
Click to Show/Hide
|
|||
| External Link | ||||
| Hu3F8 mAb | Phase 2 | [4] | ||
| External Link | ||||
| Saracatinib | Phase 2 | [5] | ||
| Synonyms |
H8H; AZD-0530; Saracatinib, AZD-0530, AZD0530; N-(5-Chloro-1,3-benzodioxol-4-yl)-7-(2-(4-methylpiperazin-1-yl)ethoxy)-5-(tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine
Click to Show/Hide
|
|||
| External Link | ||||
| ALMB-0168 | Phase 1/2 | [6] | ||
| External Link | ||||
| AU101 | Phase 1/2 | [7] | ||
| External Link | ||||
| [153Sm]CycloSam | Phase 1 | [8] | ||
| External Link | ||||
| GD2 T cells | Phase 1 | [9] | ||
| External Link | ||||
| Anti-GD2-CAR engineered T cells | Phase 1 | [10] | ||
| External Link | ||||
| Robatumumab | Discontinued in Phase 2 | [11] | ||
| External Link | ||||
| DG-8 | Investigative | [12] | ||
| Synonyms |
DH-9; PPAR gamma agonists (osteosarcoma/ADPKD); PPAR gamma agonists (osteosarcoma/ADPKD), Chinese Academy of Sciences
Click to Show/Hide
|
|||
| External Link | ||||
References