m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05202
|
[1] | |||
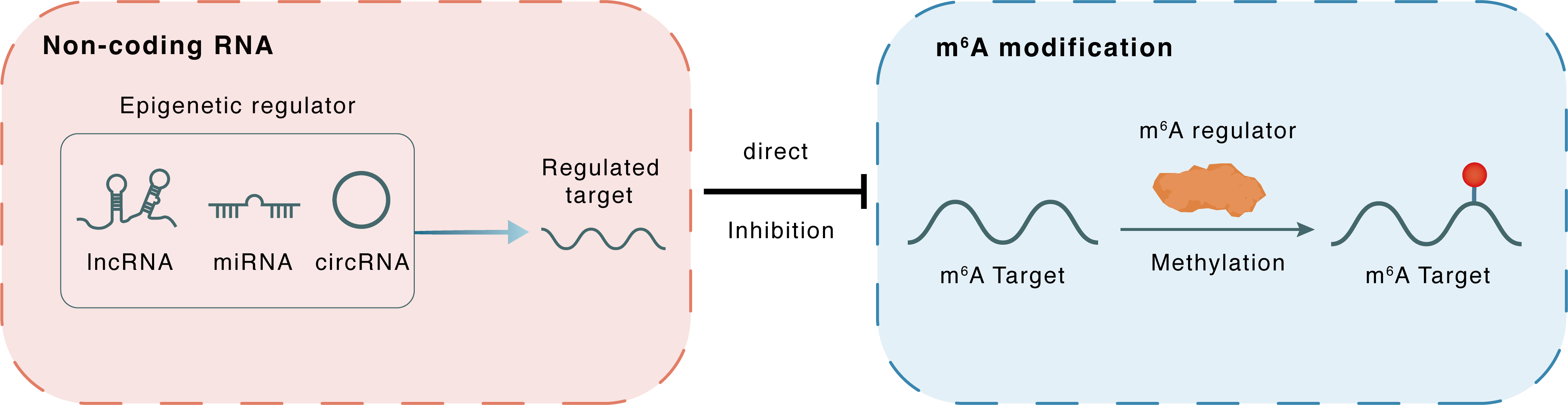 Non-coding RNA
miR-130a-3p
METTL14
lncRNA miRNA circRNA
Direct
Inhibition
m6A modification
AIFM2
AIFM2
METTL14
Methylation
Non-coding RNA
miR-130a-3p
METTL14
lncRNA miRNA circRNA
Direct
Inhibition
m6A modification
AIFM2
AIFM2
METTL14
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 14 (METTL14) | WRITER | |||
| m6A Target | Ferroptosis suppressor protein 1 (AIFM2) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa-miR-130a-3p | microRNA | View Details | ||
| Regulated Target | Methyltransferase-like protein 14 (METTL14) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Inhibition | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | Exosomal hsa-miR-130a-3p confers cisplatin resistance in esophageal cancer by regulating ferroptosis via the suppression of METTL14-mediated m6A RNA methylation of Ferroptosis suppressor protein 1 (AIFM2) | ||||
| Responsed Disease | Esophageal cancer | ICD-11: 2B70 | |||
| Responsed Drug | Cisplatin | ||||
In-vitro Model |
TE-1 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 | |
| KYSE-150 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1348 | ||
| In-vivo Model | The KYSE-150 cells were subcutaneously inoculated on the right side at a dosage of 106 cells per mouse. After the tumor grew to approximately 50 mm3, mice were randomly divided into four groups (n = 6): Control, CisR-exo, Cis, and Cis + CisR-exo. Then, normal saline or cisplatin (20 mg/kg; twice a week) was intratumorally injected alone or combined with CisR-exos (10 μ g; once every two days). | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2B70: Esophageal cancer | 15 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Pembrolizumab | Approved | [2] | ||
| External Link | ||||
| Nivolumab | Approved | [2] | ||
| External Link | ||||
| Claudiximab | Phase 3 | [3] | ||
| Synonyms |
IMAB-362; Anti-GC182 mAbs (cancer), Ganymed; Anti-GC182 monoclonal antibodies (cancer), Ganymed; Anti-CLDN18-2 mAbs (cancer), Ganymed; Anti-CLDN18-2 monoclonal antibodies (cancer), Ganymed
Click to Show/Hide
|
|||
| External Link | ||||
| Golnerminogene pradenovac | Phase 3 | [4] | ||
| Synonyms |
TNFerade (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| DKN-01 | Phase 2 | [5] | ||
| External Link | ||||
| Pegamotecan | Phase 2 | [6] | ||
| Synonyms |
Prothecan; EZ-246; PEG-camptothecin; PEG-camptothecin, Enzon; Polyethylene glycol-camptothecin, Enzon
Click to Show/Hide
|
|||
| External Link | ||||
| APR-246 | Phase 2 | [2] | ||
| Synonyms |
Eprenetapopt
Click to Show/Hide
|
|||
| External Link | ||||
| Opdivo + Yervoy | Phase 3 | [2] | ||
| External Link | ||||
| Anti-NY-ESO-1 CAR-T cells | Phase 1/2 | [7] | ||
| External Link | ||||
| Anti-MUC1 CAR-T cells | Phase 1/2 | [8] | ||
| External Link | ||||
| CAR-T Cells targeting EpCAM | Phase 1/2 | [9] | ||
| External Link | ||||
| PCA062 | Phase 1 | [2] | ||
| External Link | ||||
| Cellspan esophageal implant | Clinical trial | [2] | ||
| External Link | ||||
| PKI166 | Discontinued in Phase 2 | [10] | ||
| Synonyms |
PKI-166; CGP-75166; 187724-61-4; NVP-PKI166; CHEMBL1914653; AC1OCFE0; UNII-9RIE5HW38P; 9RIE5HW38P; SCHEMBL177814; GTPL7642; CHEMBL1963502; ZINC23255; AOB1619; PKI-75166; BDBM50358046; NCGC00387215-02; AS-16676; KB-275097; PKI-166, > 4-[4-[[(1R)-1-phenylethyl]amino]-7H-pyrrolo[4,5-e]pyrimidin-6-yl]phenol
Click to Show/Hide
|
|||
| External Link | ||||
| Ramorelix | Discontinued in Phase 1 | [11] | ||
| Synonyms |
Hoe-013
Click to Show/Hide
|
|||
| External Link | ||||
References