m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05193
|
[1] | |||
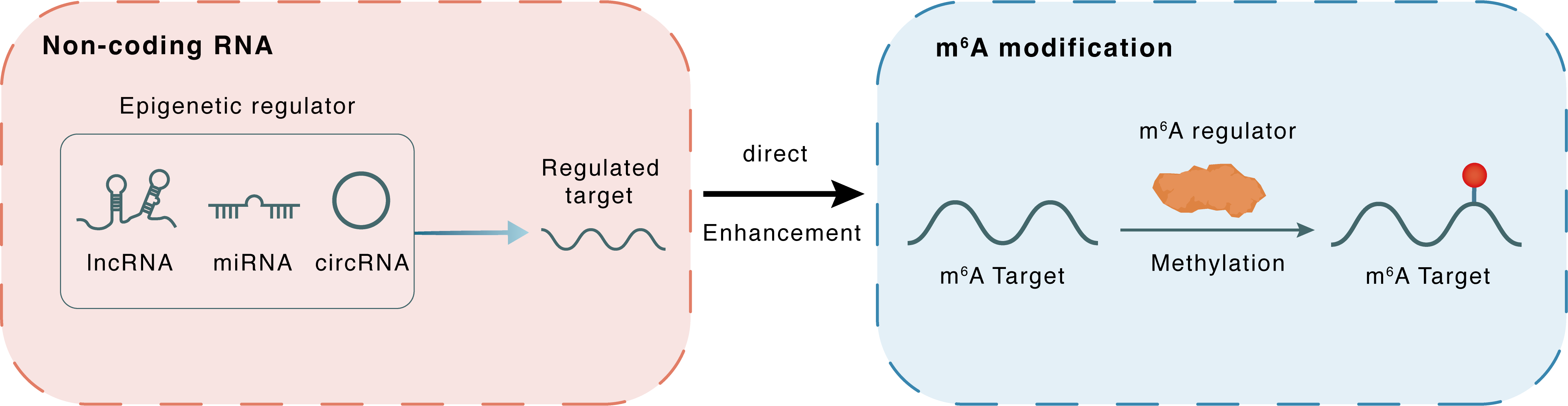 Non-coding RNA
piRNA-14633
METTL14
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
CYP1B1
CYP1B1
METTL14
Methylation
Non-coding RNA
piRNA-14633
METTL14
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
CYP1B1
CYP1B1
METTL14
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 14 (METTL14) | WRITER | |||
| m6A Target | Cytochrome P450 1B1 (CYP1B1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | piR-14633 | piRNA | View Details | ||
| Regulated Target | Methyltransferase-like protein 14 (METTL14) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | METTL14 was a directed target gene of piR-14633. Knockdown of METTL14 with siRNA attenuated proliferation, migration and invasion of CC cells. piRNA-14633 increased Cytochrome P450 1B1 (CYP1B1) expression, while silencing of METTL14 impaired its expression. | ||||
| Responsed Disease | Cervical cancer | ICD-11: 2C77 | |||
In-vitro Model |
Ca Ski | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1100 | |
| SiHa | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 | ||
| End1/E6E7 | Normal | Homo sapiens | CVCL_3684 | ||
| In-vivo Model | To examine the effects of piRNA-14633 on subcutaneous xenograft growth, BALB/c nude mice (Beijing Vital River Laboratory Animal Technology) were subcutaneously injected with 0.1 mL of cell suspension containing 2 × 106 cells. Tumor volume (mm3) was measured every 4 days using a Vernier caliper and calculated as 0.4 x (short length)2 × long length. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Cytochrome P450 1B1 (CYP1B1) | 20 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PINOCEMBRIN | Phase 2 | [2] | ||
| Synonyms |
480-39-7; (+)-Pinocembrin; (2S)-pinocembrin; Dihydrochrysin; UNII-8T7C8CH791; NSC 43318; NSC 279005; NSC 661207; 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-phenyl-, (2S)-; (S)-5,7-dihydroxyflavanone; (S)-5,7-dihydroxy-2-phenylchroman-4-one; CHEMBL399910; CHEBI:28157; (2s)-5,7-dihydroxy-2-phenyl-2,3-dihydro-4h-chromen-4-one; (S)-2,3-Dihydro-5,7-dihydroxy-2-phenyl-4H-1-benzopyran-4-one; 8T7C8CH791; Pinocembrin (6CI); 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-phenyl-, (-)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1679 nM | |||
| External Link | ||||
| NARINGENIN | Phase 1 | [2] | ||
| Synonyms |
5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one; 67604-48-2; 4',5,7-Trihydroxyflavanone; Naringenine; (+/-)-Naringenin; naringetol; 480-41-1; salipurpol; (-)-Naringenin; NARIGENIN; Salipurol; 5,7,4'-Trihydroxyflavanone; 93602-28-9; (S)-Naringenin; BE-14348A; NSC 34875; ( inverted exclamation markA)-Naringenin; CHEMBL32571; MLS000738094; MLS000028739; CHEBI:50202; Flavanone, 4',5,7-trihydroxy-; FTVWIRXFELQLPI-UHFFFAOYSA-N; NSC34875; NSC11855; MFCD00006844; SMR000059039; AK122638; NSC 11855; 4',7-Trihydroxyflavanone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| DIOSMETIN | Investigative | [2] | ||
| Synonyms |
520-34-3; Luteolin 4'-methyl ether; 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-chromen-4-one; Salinigricoflavonol; 4'-Methylluteolin; 5,7,3'-Trihydroxy-4'-methoxyflavone; UNII-TWZ37241OT; Luteolin 4-methyl ether; 5,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-benzopyrone; 3',5,7-trihydroxy-4'-methoxyflavone; CHEBI:4630; TWZ37241OT; 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chromen-4-one; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-; MFCD00017425; AK111246
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 16 nM | |||
| External Link | ||||
| ISOSAKUTANETIN | Investigative | [2] | ||
| Synonyms |
Isosakuranetin; 480-43-3; 4'-Methylnaringenin; UNII-U02X7TF8UA; naringenin 4'-methyl ether; U02X7TF8UA; CHEMBL470266; CHEBI:27552; (S)-2,3-Dihydro-5,7-dihydroxy-2-(4-methoxyphenyl)-4-benzopyrone; Citrifoliol; (S)-5,7-dihydroxy-2-(4-methoxyphenyl)chroman-4-one; (2S)-5,7-dihydroxy-2-(4-methoxyphenyl)-2,3-dihydrochromen-4-one; (2S)-5,7-dihydroxy-2-(4-methoxyphenyl)-2,3-dihydro-4H-chromen-4-one; 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(4-methoxyphenyl)-, (2S)-; 4'-Methoxy-5,7-dihydroxyflavonone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1024 nM | |||
| External Link | ||||
| KAEMPFERIDE | Investigative | [2] | ||
| Synonyms |
491-54-3; Kaempferid; 4'-Methylkaempferol; 4'-O-Methylkaempferol; Kaempferol 4'-methyl ether; Kaemperide; Campheride; Kempferide; UNII-508XL61MPD; 4'-Methoxy-3,5,7-trihydroxyflavone; NSC 407294; KAMPFERIDE; 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-(4-methoxyphenyl)-; 5,7-Dihydroxy-4'-methoxyflavonol; 3,5,7-Trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one; EINECS 207-738-4; BRN 0305378; 3,5,7-Trihydroxy-2-(4-methoxyphenyl)-4-benzopyrone; Flavone, 3,5,7-trihydroxy-4'-methoxy-; CHEMBL40919; CHEBI:6099; 508XL61MPD
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6 nM | |||
| External Link | ||||
| TAMARIXETIN | Investigative | [2] | ||
| Synonyms |
603-61-2; 4'-Methoxyquercetin; 4'-O-Methylquercetin; Quercetin 4'-methyl ether; UNII-73WRA8Z8M8; 3,3',5,7-Tetrahydroxy-4'-methoxyflavone; 4'-O-Methyl Quercetin; Quercetin-4'-methylether; 73WRA8Z8M8; CHEMBL226034; CHEBI:67492; 3,5,7-Trihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-benzopyrone; 3,5,7-trihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-chromen-4-one; 4'-Methoxy-3,3',5,7-tetrahydroxyflavone; Tamaraxetin; 3-O-rhamnopyranosyl-1-2-glucopyranoside; 3-O-alpha-L-rhamnopyranosyl-1-2-beta-D-glucopyranoside; AC1NQYX7
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| 3-[2-(3,5-Dimethoxy-phenyl)-vinyl]-furan | Investigative | [3] | ||
| Synonyms |
CHEMBL43396; SCHEMBL7047273; SCHEMBL7047277; 3-[(E)-3,5-Dimethoxystyryl]furan; ZINC13471767; BDBM50108047; AKOS015967552
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2100 nM | |||
| External Link | ||||
| ERIODICTYOL | Investigative | [2] | ||
| Synonyms |
(+/-)-Eriodictyol; 4049-38-1; MLS000877024; 5,7,3',4'-Tetrahydroxyflavanone; 2-(3,4-dihydroxyphenyl)-5,7-dihydroxychroman-4-one; 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-chroman-4-one; SMR000440624; 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-2,3-dihydro-5,7-dihydroxy-; 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-2,3-dihydrochromen-4-one; Eriodictyol, (+/-)-; Eriodicytol; ERIODYCTOL; Flavanone, 3',4',5,7-tetrahydroxy-; AC1L1WLT
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1284 nM | |||
| External Link | ||||
| HOMOERIODICTYOL | Investigative | [2] | ||
| Synonyms |
(-)-Homoeriodictyol; 446-71-9; Eriodictyonone; UNII-EHE7H3705C; Eriodictyol 3'-methyl ether; 5,7,4'-Trihydroxy-3'-methoxyflavanone; CHEMBL490170; EHE7H3705C; CHEBI:74960; (S)-2,3-Dihydro-5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4-benzopyrone; Q-100585; (+/-)-Homoeriodictyol; cyanidanon-3-methyl ether 1625; EINECS 207-173-3; AC1Q6KID; AC1L2K7J; SCHEMBL39497; DTXSID30196243; FTODBIPDTXRIGS-ZDUSSCGKSA-N; 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-; ZINC4098322; BDBM50325672
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 240 nM | |||
| External Link | ||||
| N-(2,4-Dimethoxy-phenyl)-3,5-dimethoxy-benzamide | Investigative | [3] | ||
| Synonyms |
N-(2,4-dimethoxyphenyl)-3,5-dimethoxybenzamide; CHEMBL42427; Oprea1_091011; MolPort-002-962-230; ZINC3192499; AC1M5424; BDBM50108051; STK167661; AKOS001091335; MCULE-2861059800; ST50986910
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 670 nM | |||
| External Link | ||||
| 4-[2-(3,5-Dimethoxy-phenyl)-vinyl]-pyridine | Investigative | [3] | ||
| Synonyms |
CHEMBL43013; SCHEMBL7047647; SCHEMBL7047642; BDBM50108054; ZINC13471766; AKOS015967530; 4-[(E)-3,5-Dimethoxystyryl]pyridine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 460 nM | |||
| External Link | ||||
| 2-[2-(3,5-Dimethoxy-phenyl)-vinyl]-thiophene | Investigative | [3] | ||
| Synonyms |
CHEMBL42428; SCHEMBL7042974; SCHEMBL7042968; ZINC13471769; BDBM50108048; AKOS015967551; (E)-2-(3,5-dimethoxystyryl)thiophene; 2-[(E)-3,5-Dimethoxystyryl]thiophene
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM | |||
| External Link | ||||
| CHRYSOERIOL | Investigative | [2] | ||
| Synonyms |
491-71-4; Chryseriol; Luteolin 3'-methyl ether; 3'-O-Methylluteolin; 3'-O-Methyluteolin; 5,7-Dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4-benzopyrone; 5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-chromen-4-one; 5,7-Dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-1-benzopyran-4-one; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-; UNII-Q813145M20; EINECS 207-742-6; BRN 0295004; CHEMBL214321; CHEBI:16514
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 19.7 nM | |||
| External Link | ||||
| ISORHAMNETIN | Investigative | [2] | ||
| Synonyms |
480-19-3; 3-Methylquercetin; Isorhamnetol; 3'-Methoxyquercetin; 3'-Methoxy-3,4',5,7-tetrahydroxyflavone; isorhamnetine; 3'-Methylquercetin; C.I. 75680; 3-Methylquercetine; UNII-07X3IB4R4Z; CCRIS 3791; C16H12O7; EINECS 207-545-5; Flavone, 3'-methoxy-3,4',5,7-tetrahydroxy-; BRN 0044723; 3,5,7,4'-Tetrahydroxy-3'-methoxyflavone; CHEMBL379064; CHEBI:6052; 07X3IB4R4Z
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 3 nM | |||
| External Link | ||||
| ACACETIN | Investigative | [2] | ||
| Synonyms |
480-44-4; Linarigenin; 5,7-Dihydroxy-4'-methoxyflavone; Acacetine; 4'-Methoxyapigenin; Buddleoflavonol; Linarisenin; Akatsetin; 5,7-Dihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one; Apigenin 4'-methyl ether; 5,7-Dioxy-4'-methoxyflavone; Apisenin 4'-methyl ether; Apigenin 4'-dimethyl ether; UNII-KWI7J0A2CC; NSC 76061; Flavone, 5,7-dihydroxy-4'-methoxy-; 5,7-dihydroxy-2-(4-methoxyphenyl)chromen-4-one; ACAETIN; 4'-Methoxy-5,7-dihydroxyflavone; NSC76061; EINECS 207-552-3; KWI7J0A2CC
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 7 nM | |||
| External Link | ||||
| TRISMETHOXYRESVERATROL | Investigative | [3] | ||
| Synonyms |
22255-22-7; trans-Trimethoxyresveratrol; (E)-1,3-Dimethoxy-5-(4-methoxystyryl)benzene; (E)-3,5,4'-Trimethoxystilbene; 3,4',5-trimethoxy-trans-stilbene; 3,4',5-trimethoxystilbene; 3,5,4'-trimethoxystilbene; TRIMETHOXYSTILBENE; E-Resveratrol trimethyl ether; CHEMBL296411; 1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]benzene; trans-3,4',5-trimethoxystilbene; GDHNBPHYVRHYCC-SNAWJCMRSA-N; (E)-3,4',5-Trimethoxystilbene; 5-[2-(4-Methoxyphenyl)Ethenyl]-1,3-Dimethoxy Benzene
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 790 nM | |||
| External Link | ||||
| Galangin | Investigative | [2] | ||
| Synonyms |
548-83-4; Norizalpinin; 3,5,7-Trihydroxyflavone; 3,5,7-Trihydroxy-2-phenyl-4H-chromen-4-one; 3,5,7-triOH-Flavone; UNII-142FWE6ECS; 3,5,7-Trihydroxy-2-phenyl-4-benzopyrone; 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-phenyl-; EINECS 208-960-4; NSC407229; FLAVONE, 3,5,7-TRIHYDROXY-; NSC 407229; NSC-407229; 4H-Benzopyran-4-one, 3,5,7-trihydroxy-2-phenyl-; BRN 0272179; 142FWE6ECS; 3,5,7-trihydroxy-2-phenylchromen-4-one; CHEBI:5262; CHEMBL309490; VCCRNZQBSJXYJD-UHFFFAOYSA-N; 3,5,7-trihydroxy-2-phenyl-4H-benzopyran-4-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 25 nM | |||
| External Link | ||||
| Chrysin | Investigative | [2] | ||
| Synonyms |
480-40-0; 5,7-Dihydroxyflavone; Chrysine; 5,7-Dihydroxy-2-phenyl-4H-chromen-4-one; Crysin; 5,7-dihydroxy-2-phenylchromen-4-one; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-phenyl-; UNII-3CN01F5ZJ5; NSC-407436; FLAVONE, 5,7-DIHYDROXY-; EINECS 207-549-7; NSC407436; 5,7-Dihydroxy-2-phenyl-4H-1-benzopyran-4-one; CHEMBL117; NSC 407436; 5,7-Dihydroxy-2-phenyl-chromen-4-one; BRN 0233276; 3CN01F5ZJ5; CHEBI:75095; RTIXKCRFFJGDFG-UHFFFAOYSA-N; 5,7-Dihydroxy-2-phenyl-4H-benzo(b)pyran-4-one; MFCD00006834; Chrysin, 99+%; CAS-480-40-0
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 16 nM | |||
| External Link | ||||
| APIGENIN | Investigative | [2] | ||
| Synonyms |
520-36-5; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one; Chamomile; Versulin; Spigenin; Apigenol; 4',5,7-Trihydroxyflavone; Apigenine; C.I. Natural Yellow 1; 5,7,4'-Trihydroxyflavone; Pelargidenon 1449; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone; 2-(p-Hydroxyphenyl)-5,7-dihydroxychromone; UCCF 031; NSC 83244; UNII-7V515PI7F6; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one; CCRIS 3789; CHEBI:18388; CHEMBL28; EINECS 208-292-3; 4H-1-Benzopyran-4-one, 5,7-di
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 64 nM | |||
| External Link | ||||
| KAEMPFEROL | Investigative | [2] | ||
| Synonyms |
520-18-3; Kaempherol; Kempferol; Trifolitin; Populnetin; Robigenin; Rhamnolutein; Pelargidenolon; Rhamnolutin; Swartziol; Indigo Yellow; Kampherol; Nimbecetin; Kampferol; Campherol; Kaemferol; 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one; 5,7,4'-Trihydroxyflavonol; Pelargidenolon 1497; 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; C.I. 75640; CCRIS 41; NSC 407289; 3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one; 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-(4-hydroxyphe
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 43 nM | |||
| External Link | ||||
References