m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03189
|
[1] | |||
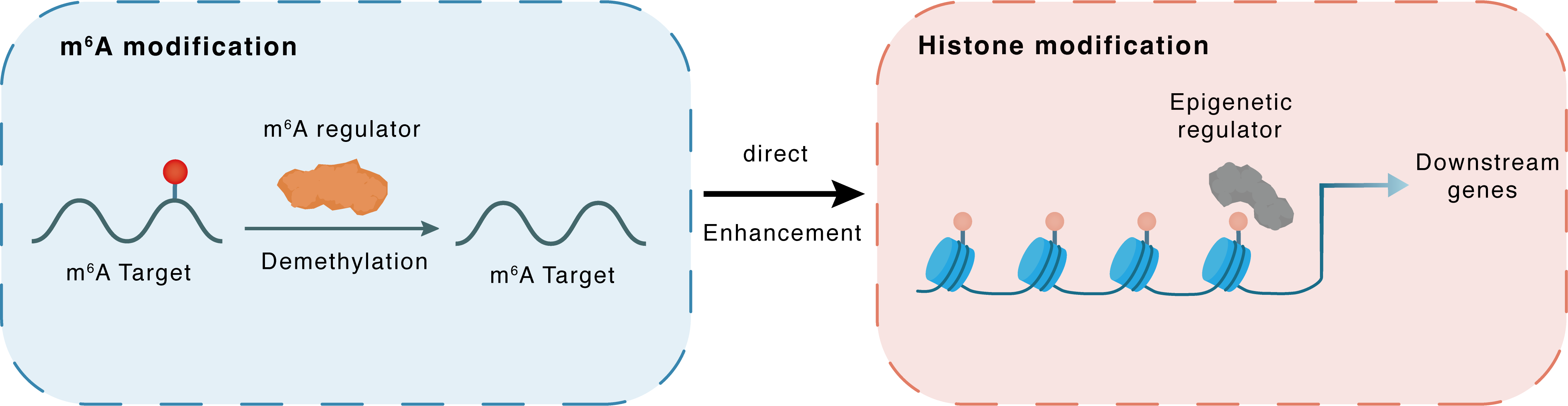 m6A modification
HDAC4
HDAC4
ALKBH5
Demethylation
m6A modification
HDAC4
HDAC4
ALKBH5
Demethylation
 : m6A sites
Direct
Enhancement
Histone modification
FOXO3
HDAC4
Downstream Gene : m6A sites
Direct
Enhancement
Histone modification
FOXO3
HDAC4
Downstream Gene
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | RNA demethylase ALKBH5 (ALKBH5) | ERASER | |||
| m6A Target | Histone deacetylase 4 (HDAC4) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone deacetylase 4 (HDAC4) | ERASER | View Details | ||
| Regulated Target | Forkhead box protein O3 (FOXO3) | View Details | |||
| Crosstalk Relationship | m6A → Histone modification | Enhancement | |||
| Crosstalk Mechanism | m6A modification impacts directly histone modification through modulating the expression level of histone-associated enzymes | ||||
| Crosstalk Summary | ALKBH5 demethylates and stabilizes Hdac4 mRNA. Histone deacetylase 4 (HDAC4) interacts with and deacetylates Forkhead box protein O3 (FOXO3), resulting in a significant increase in FoxO3 expression | ||||
| Responsed Disease | Progressive muscular atrophy | ICD-11: 8B60.3 | |||
| Pathway Response | FoxO signaling pathway | hsa04068 | |||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Histone deacetylase 4 (HDAC4) | 104 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PMID29671355-Compound-52 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 55 nM | |||
| External Link | ||||
| PMID29671355-Compound-72a | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| PMID29671355-Compound-47c | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 40 nM | |||
| External Link | ||||
| PMID29671355-Compound-70 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| PMID29671355-Compound-71b | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8 nM | |||
| External Link | ||||
| PMID29671355-Compound-72b | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21 nM | |||
| External Link | ||||
| PMID29671355-Compound-47a | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| PMID29671355-Compound-50 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 12 nM | |||
| External Link | ||||
| PMID29671355-Compound-47b | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| PMID29671355-Compound-71a | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| PMID29671355-Compound-47d | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 40 nM | |||
| External Link | ||||
| PMID29671355-Compound-12 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 120 nM | |||
| External Link | ||||
| PMID29671355-Compound-68b | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.74 nM | |||
| External Link | ||||
| PMID29671355-Compound-45a | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 49 nM | |||
| External Link | ||||
| PMID29671355-Compound-61 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1000000 nM | |||
| External Link | ||||
| PMID29671355-Compound-23 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3820 nM | |||
| External Link | ||||
| PMID29671355-Compound-56 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 9220 nM | |||
| External Link | ||||
| PMID29671355-Compound-67 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2840 nM | |||
| External Link | ||||
| PMID29671355-Compound-31 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1970 nM | |||
| External Link | ||||
| PMID29671355-Compound-21 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 16400 nM | |||
| External Link | ||||
| PMID29671355-Compound-62 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-43 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 100000 nM | |||
| External Link | ||||
| PMID29671355-Compound-25 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3310 nM | |||
| External Link | ||||
| N-hydroxy-9,10-dihydroanthracene-9-carboxamide | Investigative | [3] | ||
| Synonyms |
CHEMBL575482; SCHEMBL4541357
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2320 nM | |||
| External Link | ||||
| N-hydroxy-2,2-diphenylacetamide | Investigative | [3] | ||
| Synonyms |
Diphenylacetohydroxamic acid; 4099-51-8; N-Hydroxy diphenylacetamide; CHEMBL396097; NSC44620; benzeneacetamide, n-hydroxy-; A-phenyl-; N-Hydroxydiphenylacetamide; BENZENEACETAMIDE,N-HYDROXY-A-PHENYL-; AC1Q5QC3; SCHEMBL2839032; CTK8I6435; DTXSID10286297; AC1L6390; ZINC4522248; NSC-44620; BDBM50207561; MFCD16314231; AKOS022308585; Diphenylacetohydroxamic acid, >
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 250 nM | |||
| External Link | ||||
| N-hydroxy-9H-xanthene-9-carboxamide | Investigative | [3] | ||
| Synonyms |
CHEMBL583490; 9H-Xanthene-9-carboxamide,N-hydroxy-; SCHEMBL2843958; BDBM50300446
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 250 nM | |||
| External Link | ||||
| 2,2-bis(3-fluorophenyl)-N-hydroxyacetamide | Investigative | [3] | ||
| Synonyms |
CHEMBL574594; SCHEMBL4536216
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 700 nM | |||
| External Link | ||||
| AZUMAMIDE E | Investigative | [4] | ||
| Synonyms |
CHEMBL402363
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2280 nM | |||
| External Link | ||||
| 2,2-bis(4-fluorophenyl)-N-hydroxyacetamide | Investigative | [3] | ||
| Synonyms |
CHEMBL573190; SCHEMBL2841871
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1100 nM | |||
| External Link | ||||
| N-hydroxy-2,2-diphenylpropanamide | Investigative | [3] | ||
| Synonyms |
CHEMBL585365; SCHEMBL2844474
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4500 nM | |||
| External Link | ||||
| AZUMAMIDE B | Investigative | [4] | ||
| Synonyms |
CHEMBL402727
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3660 nM | |||
| External Link | ||||
| 2,2-bis(4-chlorophenyl)-N-hydroxyacetamide | Investigative | [3] | ||
| Synonyms |
CHEMBL572805; SCHEMBL2848402
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 960 nM | |||
| External Link | ||||
| AZUMAMIDE C | Investigative | [4] | ||
| Synonyms |
CHEMBL257972
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3160 nM | |||
| External Link | ||||
| LARGAZOLE | Investigative | [5] | ||
| Synonyms |
CHEMBL1173445; (+)-Largazole; SCHEMBL71330; ZINC56861395
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3000 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-L-A1in-L-Ala-D-Pro-) | Investigative | [6] | ||
| Synonyms |
CHEMBL393260
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 25 nM | |||
| External Link | ||||
| 7-mercapto-N-(4-phenylthiazol-2-yl)heptanamide | Investigative | [7] | ||
| Synonyms |
CHEMBL419758; NCH-31; JMC505425 Compound 7; BDBM19131; 7-mercapto-N-(4-phenyl-2-thiazolyl)heptanamide; N-(4-phenyl-1,3-thiazol-2-yl)-7-sulfanylheptanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 32 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-D-A1in-L-Ala-D-Pro-) | Investigative | [6] | ||
| Synonyms |
CHEMBL390991
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.4 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-A2in-L-Ala-D-Pro-) | Investigative | [6] | ||
| Synonyms |
CHEMBL394261
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1.6 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ph5-D-Pro-) | Investigative | [6] | ||
| Synonyms |
CHEMBL391384
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.7 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-D-2MePhe-L-Ala-D-Pro-) | Investigative | [6] | ||
| Synonyms |
CHEMBL393261
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 70 nM | |||
| External Link | ||||
| santacruzamate A | Investigative | [8] | ||
| Synonyms |
CAY10683
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 > 1000 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ser(Bzl)-D-Pro-) | Investigative | [6] | ||
| Synonyms |
CHEMBL241555
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1.6 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Phg-D-Pro-) | Investigative | [6] | ||
| Synonyms |
CHEMBL428737
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 56 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ala-D-Tic-) | Investigative | [6] | ||
| Synonyms |
CHEMBL238587
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4.2 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ala-D-Pro-) | Investigative | [6] | ||
| Synonyms |
CHEMBL238596; BDBM50222727
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5.2 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ser-D-Pro-) | Investigative | [6] | ||
| Synonyms |
CHEMBL393961
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ph4-D-Pro-) | Investigative | [6] | ||
| Synonyms |
CHEMBL391383
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3.2 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-L-2MePhe-L-Ala-D-Pro-) | Investigative | [6] | ||
| Synonyms |
CHEMBL393464
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.2 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Phe-D-Pro-) | Investigative | [6] | ||
| Synonyms |
CHEMBL238829; BDBM50222732
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1.8 nM | |||
| External Link | ||||
| TMP269 | Investigative | [9] | ||
| Synonyms |
TMFO1; compound 1 [PMID: 23524983]; TMP-269; TMP 269
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 90.5 nM | |||
| External Link | ||||
| 8-Oxo-8-phenyl-octanoic acid hydroxyamide | Investigative | [10] | ||
| Synonyms |
CHEMBL95959; SCHEMBL3383197; N-hydroxy-8-oxo-8-phenyloctanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ST-3050 | Investigative | [11] | ||
| Synonyms |
CHEMBL472631; SCHEMBL3445133; SCHEMBL3445139; BDBM50278222
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 16100 nM | |||
| External Link | ||||
| Octanedioic acid bis-hydroxyamide | Investigative | [12] | ||
| Synonyms |
Suberohydroxamic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 9,9,9-Trifluoro-8-oxo-nonanoic acid phenylamide | Investigative | [10] | ||
| Synonyms |
9,9,9-Trifluoro-8-Oxo-N-Phenylnonanamide; CHEMBL113537; 2gh6; SCHEMBL2702892; KRCXZGYVOZSCSF-UHFFFAOYSA-N; BDBM50121062; DB07553
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 5.1 nM | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid phenylamide | Investigative | [13] | ||
| Synonyms |
Thiol-SAHA (t-SAHA); CHEMBL325676; SCHEMBL14821761; BDBM152692
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-benzenesulfinylhexanoic acid hydroxamide | Investigative | [14] | ||
| Synonyms |
6-(benzenesulfinyl)hexanoic acid hydroxyamide; 875737-03-4
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-Mercapto-ethyl)-N'-phenyl-succinamide | Investigative | [15] | ||
| Synonyms |
CHEMBL193959
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid biphenyl-4-ylamide | Investigative | [13] | ||
| Synonyms |
CHEMBL112311
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-phenylacetylamino-benzamide | Investigative | [16] | ||
| Synonyms |
CHEMBL356824; 656261-23-3; SCHEMBL675578; CTK1J6158; DTXSID40458440; ZINC13533297; AKOS030583151; Benzeneacetamide, N-[4-[(hydroxyamino)carbonyl]phenyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-(2-Bromo-acetylamino)-hexanoic acid phenylamide | Investigative | [13] | ||
| Synonyms |
CHEMBL344920; 651767-99-6; SCHEMBL3736839; CTK1J8444; DTXSID50432973; HWYLREOMBVUGJQ-UHFFFAOYSA-N; BDBM50222416; ZINC13587789; AKOS030603042; N-Phenyl-6-(bromoacetylamino)hexanamide; Hexanamide, 6-[(bromoacetyl)amino]-N-phenyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(phenylacetylamino-methyl)-benzamide | Investigative | [17] | ||
| Synonyms |
CHEMBL143674; SCHEMBL673760
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-4-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [18] | ||
| Synonyms |
CHEMBL126355; BDBM50222394
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Octanedioic acid hydroxyamide pyridin-4-ylamide | Investigative | [19] | ||
| Synonyms |
SCHEMBL8082656; CHEMBL165162; ZINC13472304
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(6-Mercapto-hexyl)-benzamide | Investigative | [13] | ||
| Synonyms |
CHEMBL112364; BDBM50223650
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Hydroxy-N-(5-hydroxycarbamoyl-pentyl)-benzamide | Investigative | [19] | ||
| Synonyms |
CHEMBL167455
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-((R)-2-phenyl-butyrylamino)-benzamide | Investigative | [16] | ||
| Synonyms |
SCHEMBL675474
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-benzenesulfonylhexanoic acid hydroxamide | Investigative | [14] | ||
| Synonyms |
CHEMBL203207
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 9-(Biphenyl-4-yloxy)-1,1,1-trifluoro-nonan-2-one | Investigative | [10] | ||
| Synonyms |
SCHEMBL7373122; CHEMBL116578
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Thioacetic acid S-(6-phenylcarbamoyl-hexyl) ester | Investigative | [13] | ||
| Synonyms |
CHEMBL111806; SCHEMBL14812153
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Butyrylamino-N-hydroxy-benzamide | Investigative | [17] | ||
| Synonyms |
CHEMBL142254; 656261-22-2; Benzamide, N-hydroxy-4-[(1-oxobutyl)amino]-; SCHEMBL675234; CTK1J6159; DTXSID90461262
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Naphthalen-2-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [18] | ||
| Synonyms |
CHEMBL127328
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid biphenyl-3-ylamide | Investigative | [13] | ||
| Synonyms |
CHEMBL320323
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione | Investigative | [20] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(2-phenyl-butyrylamino)-benzamide | Investigative | [16] | ||
| Synonyms |
SCHEMBL676079
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Dimethylamino-N-(6-mercapto-hexyl)-benzamide | Investigative | [13] | ||
| Synonyms |
CHEMBL324126
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid pyridin-3-ylamide | Investigative | [13] | ||
| Synonyms |
CHEMBL332246; Heptanamide, 7-mercapto-N-3-pyridinyl-; BDBM50223653
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-Phenoxy-hexane-1-thiol | Investigative | [13] | ||
| Synonyms |
CHEMBL109796; 6-phenoxyhexane-1-thiol; 1-Hexanethiol, 6-phenoxy-; SCHEMBL5679745; MolPort-020-180-823; BDBM50223652; AKOS018584222; MCULE-9521857089
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Benzoylamino-N-hydroxy-benzamide | Investigative | [16] | ||
| Synonyms |
SCHEMBL673678; CHEMBL191227
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Chloro-N-(5-hydroxycarbamoyl-pentyl)-benzamide | Investigative | [10] | ||
| Synonyms |
CHEMBL143734; NSC718168; AC1L8L82; SCHEMBL13039735; ZINC5579677; BDBM50082664; NSC-718168; NCI60_040737; 6-(4-Chlorobenzoylamino)hexanehydroxamic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-(Biphenyl-4-yloxy)-1,1,1-trifluoro-octan-2-one | Investigative | [18] | ||
| Synonyms |
CHEMBL112148; SCHEMBL7364383; BDBM50218532
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-3-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [18] | ||
| Synonyms |
CHEMBL127351; SCHEMBL7365180; HWZHDGRMABBYOV-UHFFFAOYSA-N; BDBM50222367; 7-((1,1'-biphenyl)-3-yloxy)-1-(1 ,3-oxazol-2-yl)-1-heptanone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-Mercapto-hexanoic acid phenylamide | Investigative | [13] | ||
| Synonyms |
CHEMBL109654; Hexanamide, 6-mercapto-N-phenyl-; SCHEMBL14254925; BDBM50027600
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclostellettamine derivative | Investigative | [21] | ||
| Synonyms |
CHEMBL88332
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(5-Hydroxycarbamoyl-pentyl)-4-nitro-benzamide | Investigative | [10] | ||
| Synonyms |
CHEMBL139999; SCHEMBL1232700; BDBM50082661; ZINC13472309
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-Mercapto-pentanoic acid phenylamide | Investigative | [13] | ||
| Synonyms |
N-Phenyl-5-mercaptovaleramide; CHEMBL114344; Pentanamide, 5-mercapto-N-phenyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Octanedioic acid hydroxyamide pyridin-2-ylamide | Investigative | [19] | ||
| Synonyms |
SCHEMBL8090513; CHEMBL164872; ZINC13472303
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-Mercapto-ethyl)-N'-phenyl-oxalamide | Investigative | [15] | ||
| Synonyms |
CHEMBL193979
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(methylsulfonylthio)ethyl 2-propylpentanoate | Investigative | [20] | ||
| Synonyms |
CHEMBL271677; SCHEMBL4156413
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (E)-8-Biphenyl-4-yl-1-oxazol-2-yl-oct-7-en-1-one | Investigative | [18] | ||
| Synonyms |
CHEMBL126465; SCHEMBL7368197; SCHEMBL7368201
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-((S)-2-phenyl-butyrylamino)-benzamide | Investigative | [16] | ||
| Synonyms |
SCHEMBL676080
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(3-phenyl-propionylamino)-benzamide | Investigative | [16] | ||
| Synonyms |
N-hydroxy-4-(3-phenylpropanamido)benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(5-phenyl-pentanoylamino)-benzamide | Investigative | [16] | ||
| Synonyms |
SCHEMBL7311087
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-Oxo-8-phenyl-octanoic acid | Investigative | [19] | ||
| Synonyms |
8-Oxo-8-phenyloctanoic acid; 7-Benzoylheptanoic acid; 24314-23-6; Benzeneoctanoic acid, h-oxo-; 7-BENZOYL HEPTANOIC ACID; AC1L6TSB; SCHEMBL3381106; 8-keto-8-phenyl-caprylic acid; CHEMBL162423; 8-Oxo-8-phenyloctanoic acid #; CTK4F3363; DTXSID40305602; UMCSRRHQLAVYRS-UHFFFAOYSA-N; ZINC2168376; 7009f; NSC171230; AKOS016022495; NSC-171230; MCULE-7202530747; ACM24314236; ST50825837
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(4-phenyl-butyrylamino)-benzamide | Investigative | [16] | ||
| Synonyms |
CHEMBL143336; 656261-24-4; SCHEMBL674421; CTK1J6157; DTXSID30433908; ZINC13533300; AKOS030583673; n-hydroxy-4-(4-phenylbutyryl-amino)benzamide; Benzenebutanamide, N-[4-[(hydroxyamino)carbonyl]phenyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-phenylsulfanylhexanoic acid hydroxamide | Investigative | [14] | ||
| Synonyms |
Hexanamide, N-hydroxy-6-(phenylthio)-; CHEMBL203028; SCHEMBL7317658
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ST-2987 | Investigative | [11] | ||
| Synonyms |
CHEMBL471042; SCHEMBL3444989; SCHEMBL3444984; BDBM50278220
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2040 nM | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid quinolin-3-ylamide | Investigative | [13] | ||
| Synonyms |
CHEMBL112234
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-(4-Chloro-phenyl)-pentanoic acid hydroxyamide | Investigative | [22] | ||
| Synonyms |
CHEMBL84288
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-Mercapto-octanoic acid phenylamide | Investigative | [13] | ||
| Synonyms |
8-mercapto-N-phenyloctanamide; CHEMBL326433; ZINC13609343
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(6-Hydroxycarbamoyl-hexyl)-benzamide | Investigative | [19] | ||
| Synonyms |
CHEMBL57107; 174664-71-2; SCHEMBL573254; CTK0A7470; DTXSID00433435; BDBM50220823; ZINC13490043; 7-(Benzoylamino)heptanehydroxamic acid; AKOS030580013; Benzamide, N-[7-(hydroxyamino)-7-oxoheptyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-4-yloxy)-1,1,1-trifluoro-heptan-2-one | Investigative | [10] | ||
| Synonyms |
CHEMBL326529; SCHEMBL7365237; BDBM50217957
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid benzothiazol-2-ylamide | Investigative | [13] | ||
| Synonyms |
CHEMBL178779
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(pentanoylamino-methyl)-benzamide | Investigative | [17] | ||
| Synonyms |
CHEMBL143102
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PSAMMAPLIN A | Investigative | [10] | ||
| Synonyms |
110659-91-1; Bisprasin; NSC614495; AC1O46WI; SCHEMBL364511; ZINC150352860; NSC-614495; B723735K022; J-002461; Benzenepropanamide, N,N'-(dithiodi-2,1-ethanediyl)bis(3-bromo-4-hydroxy-alpha-(hydroxyimino)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8B60: Motor neuron disease | 70 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| AEN-100 | Phase 4 | [23] | ||
| Synonyms |
Nobeldin; Nobelzin; Wilzin; NPC-02; Wilson's disease therapeutics, Nobel Pharma; Zinc acetate (Wilson's disease); Zinc acetate (Wilson's disease), Nobel Pharma/Orphan Europe/Teva; Zinc acetate (gastroretentive/ sustained-release/ tablet, amyotrophic lateral sclerosis), Adeona Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| Creatine ALS-08 | Phase 3 | [24] | ||
| Synonyms |
Creatine; ALS-02; ALS-05; ALS-08; HD-02; PD-02; PD-04; PD-06; Creatine (ALS), Avicena; Creatine (amyotrophic lateral sclerosis), Avicena; PD-01, Avicena; AL-02 (ALS), Avicena; AL-08 (ALS), Avicena; Creatine (Huntington's disease), Avicena; Creatine (Parkinson's disease), Avicena
Click to Show/Hide
|
|||
| External Link | ||||
| NT-KO-003 | Phase 2 | [25] | ||
| Synonyms |
ATH-012; NT-K0-003
Click to Show/Hide
|
|||
| External Link | ||||
| CC-100 | Phase 1 | [26] | ||
| Synonyms |
Fendosal; 53597-27-6; 5-(4,5-Dihydro-2-phenyl-3H-benz[e]indol-3-yl)salicylic acid; Alnovin; Fendosalum; HP 129; Fendosalum [INN-Latin]; UNII-9Z709558TZ; P 71-0129; C25H19NO3; NSC 351522; BRN 1665211; 2-hydroxy-5-(2-phenyl-4,5-dihydrobenzo[e]indol-3-yl)benzoic acid; 2-Hydroxy-5-(2-phenyl-4,5-dihydro-3H-benzo[e]indol-3-yl)benzoic acid; 3-(3-Carboxy-4-hydroxyphenyl)-2-phenyl-4,5-dihydro-3H-benz(e)indole; 5-(4,5-Dihydro-2-phenyl-3H-benz(e)indol-3-yl)-2-hydroxybenzoic acid; NSC351522; HP-129; 9Z709558TZ
Click to Show/Hide
|
|||
| External Link | ||||
| ISIS-SOD1 | Phase 1 | [27] | ||
| External Link | ||||
| RG-7010 | Phase 1 | [28] | ||
| Synonyms |
R-7010; PEGylated IGF1 (amyotrophic lateral sclerosis), Roche
Click to Show/Hide
|
|||
| External Link | ||||
| ORG-9273 | Discontinued in Phase 2 | [29] | ||
| Synonyms |
1-[(2beta,3alpha,5alpha,16beta,17beta)-17-Acetoxy-3-hydroxy-2-morpholinoandrostan-16-yl]-1-methylpiperidinium bromide; 17beta-Acetoxy-3alpha-hydroxy-16beta-(1-methylhexahydropyridinio-1-yl)-2beta-morpholino-5alpha-androstane bromide
Click to Show/Hide
|
|||
| External Link | ||||
| ANQ-9040 | Discontinued in Phase 1 | [30] | ||
| Synonyms |
3alpha-Acetoxy-3beta-(hexahydroazepinomethyl)-17a,17a-dimethyl-17-homo-17a-azonia-5alpha-androstane benzenesulfonate
Click to Show/Hide
|
|||
| External Link | ||||
| Celastrol | Preclinical | [31] | ||
| Synonyms |
Tripterin; Tripterine; Celastrol, Celastrus scandens; (2R,4aS,6aR,6aS,14aS,14bR)-10-hydroxy-2,4a,6a,6a,9,14a-hexamethyl-11-oxo-1,3,4,5,6,13,14,14b-octahydropicene-2-carboxylic acid; (2R,4aS,6aS,12bR,14aS,14bR)-10-hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydropicene-2-carboxylic acid; 3-Hydroxy-24-nor-2-oxo-1(10),3,5,7-friedelatetraen-29-oic Acid
Click to Show/Hide
|
|||
| External Link | ||||
| PLD-180 | Terminated | [32] | ||
| External Link | ||||
| AX-205 | Terminated | [33] | ||
| External Link | ||||
| TDI-0111 | Investigative | [34] | ||
| External Link | ||||
| TDI-0020 | Investigative | [34] | ||
| External Link | ||||
| TDI-0046 | Investigative | [34] | ||
| External Link | ||||
| Stem cell-derived astrocytes | Investigative | [34] | ||
| Synonyms |
Stem cell-derived astrocytes (ALS)
Click to Show/Hide
|
|||
| External Link | ||||
| TDI-0113 | Investigative | [34] | ||
| External Link | ||||
| TDI-0098 | Investigative | [34] | ||
| External Link | ||||
| Cis-tetracosenoyl sulfatide | Investigative | [34] | ||
| Synonyms |
Cis-tetracosenoyl sulfatide (amyotrophic lateral sclerosis); Cis-tetracosenoyl sulfatide (amyotrophic lateral sclerosis), Glycoregimmune
Click to Show/Hide
|
|||
| External Link | ||||
| TDI-0115 | Investigative | [34] | ||
| External Link | ||||
| TDI-0043 | Investigative | [34] | ||
| External Link | ||||
| TDI-0107 | Investigative | [35] | ||
| Synonyms |
Gene therapy (SOD1 gene inhibitor), ALS Therapy Development Foundation
Click to Show/Hide
|
|||
| External Link | ||||
| NNZ-4945 | Investigative | [34] | ||
| Synonyms |
Nerve regeneration program, Neuren/Metabolic Pharmaceuticals; Neurodegenerative disease peptides, Neuren/Metabolic Pharmaceuticals; Neuronal regeneration peptides (amyotrophic lateral sclerosis), CuroNZ; Neuronal regeneration peptides (motor neuron disease), CuroNZ; Neuronal regeneration peptides, Neuren/Calzada
Click to Show/Hide
|
|||
| External Link | ||||
| NT-FV-007 | Investigative | [34] | ||
| External Link | ||||
| TDI-0103 | Investigative | [34] | ||
| External Link | ||||
| TDI-0114 | Investigative | [34] | ||
| External Link | ||||
| SCLERON | Investigative | [34] | ||
| Synonyms |
Motoneuron degeneration blocker (amyotrophic lateral sclerosis), Lascco
Click to Show/Hide
|
|||
| External Link | ||||
| TDI-0047 | Investigative | [34] | ||
| External Link | ||||
| TDI-0033 | Investigative | [36] | ||
| Synonyms |
Gene therapy program (NGF receptor agonist); TDI-0034; TDI-0035; TDI-0036; TDI-0038; TDI-0041; Gene therapy program (NGF receptor agonist), ALS Therapy Development Foundation
Click to Show/Hide
|
|||
| External Link | ||||
| GEM-ALS | Investigative | [34] | ||
| Synonyms |
Amyotrophic lateral sclerosis therapy (conjugated protein), Gemac
Click to Show/Hide
|
|||
| External Link | ||||
| TDI-0042 | Investigative | [34] | ||
| External Link | ||||
| NsG-33 | Investigative | [34] | ||
| Synonyms |
Meteorin
Click to Show/Hide
|
|||
| External Link | ||||
| TDI-0066 | Investigative | [34] | ||
| External Link | ||||
| ETN-002 | Investigative | [34] | ||
| External Link | ||||
| TDI-0010 | Investigative | [34] | ||
| External Link | ||||
| TDI-0105 | Investigative | [34] | ||
| External Link | ||||
| TDI-0029 | Investigative | [34] | ||
| External Link | ||||
| TDI-0049 | Investigative | [34] | ||
| External Link | ||||
| TDI-0068 | Investigative | [34] | ||
| External Link | ||||
| AAV-IGF | Investigative | [34] | ||
| Synonyms |
AAV-IGF (amyotrophic lateral sclerosis); AAV1-IGF-1; AAV-IGF (amyotrophic lateral sclerosis), Genzyme
Click to Show/Hide
|
|||
| External Link | ||||
| TDI-0048 | Investigative | [34] | ||
| External Link | ||||
| TDI-0099 | Investigative | [34] | ||
| External Link | ||||
| TDI-0074 | Investigative | [34] | ||
| External Link | ||||
| TDI-0060 | Investigative | [35] | ||
| External Link | ||||
| TDI-0087 | Investigative | [34] | ||
| External Link | ||||
| TDI-0026 | Investigative | [34] | ||
| External Link | ||||
| TDI-0090 | Investigative | [34] | ||
| External Link | ||||
| TDI-0039 | Investigative | [34] | ||
| External Link | ||||
| TDI-0053 | Investigative | [34] | ||
| External Link | ||||
| TDI-0018 | Investigative | [34] | ||
| External Link | ||||
| TDI-0032 | Investigative | [34] | ||
| External Link | ||||
| TDI-0067 | Investigative | [34] | ||
| External Link | ||||
| CERE-135 | Investigative | [34] | ||
| Synonyms |
CERE-130; Insulin like growth factor-1 gene therapy, Ceregene; IGF-1 gene therapy (ALS), Ceregene; IGF-1 gene therapy (amyotrophic lateral sclerosis), Ceregene; AAV-IGF-1 gene therapy (ALS), Ceregene
Click to Show/Hide
|
|||
| External Link | ||||
| TDI-0028 | Investigative | [34] | ||
| Synonyms |
ALS-TDI-00846
Click to Show/Hide
|
|||
| External Link | ||||
| TDI-0094 | Investigative | [34] | ||
| External Link | ||||
| ORG-9453 | Investigative | [34] | ||
| Synonyms |
1-[3alpha-Acetoxy-17beta-butyryloxy-2beta-(piperidin-1-yl)-5alpha-androstan-16beta-yl]-1-methylpiperidinium bromide
Click to Show/Hide
|
|||
| External Link | ||||
| TDI-0025 | Investigative | [34] | ||
| External Link | ||||
| TDI-0079 | Investigative | [35] | ||
| Synonyms |
Superoxide dismutase-1 (SOD1) gene modulator (amyotrophic lateral sclerosis), ALS Therapy Development Foundation
Click to Show/Hide
|
|||
| External Link | ||||
| RTA-801 | Investigative | [34] | ||
| External Link | ||||
| TDI-0055 | Investigative | [34] | ||
| External Link | ||||
| TDI-0054 | Investigative | [34] | ||
| External Link | ||||
| TDI-0059 | Investigative | [36] | ||
| External Link | ||||
| TDI-0102 | Investigative | [34] | ||
| External Link | ||||
| TDI-0106 | Investigative | [34] | ||
| External Link | ||||
| TDI-0015 | Investigative | [34] | ||
| External Link | ||||
| TDI-0050 | Investigative | [34] | ||
| External Link | ||||
| MGN-8107 | Investigative | [34] | ||
| Synonyms |
MiR-206 targeted therapeutic (amyotrophic lateral sclerosis), miRagen Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| TDI-0065 | Investigative | [34] | ||
| External Link | ||||
| TDI-0057 | Investigative | [34] | ||
| Synonyms |
Gene therapy (cellular stress), ALS Therapy Development Foundation
Click to Show/Hide
|
|||
| External Link | ||||
| ALS-AAV9 | Investigative | [34] | ||
| Synonyms |
Amyotrophic lateral sclerosis gene therapy, RegenX Biosciences; Adeno-associated virus vector-9 based gene therapy (injectable, ALS), RegenX Bioscience
Click to Show/Hide
|
|||
| External Link | ||||
| TDI-0051 | Investigative | [34] | ||
| External Link | ||||
References