m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT02272
|
[1], [2] | |||
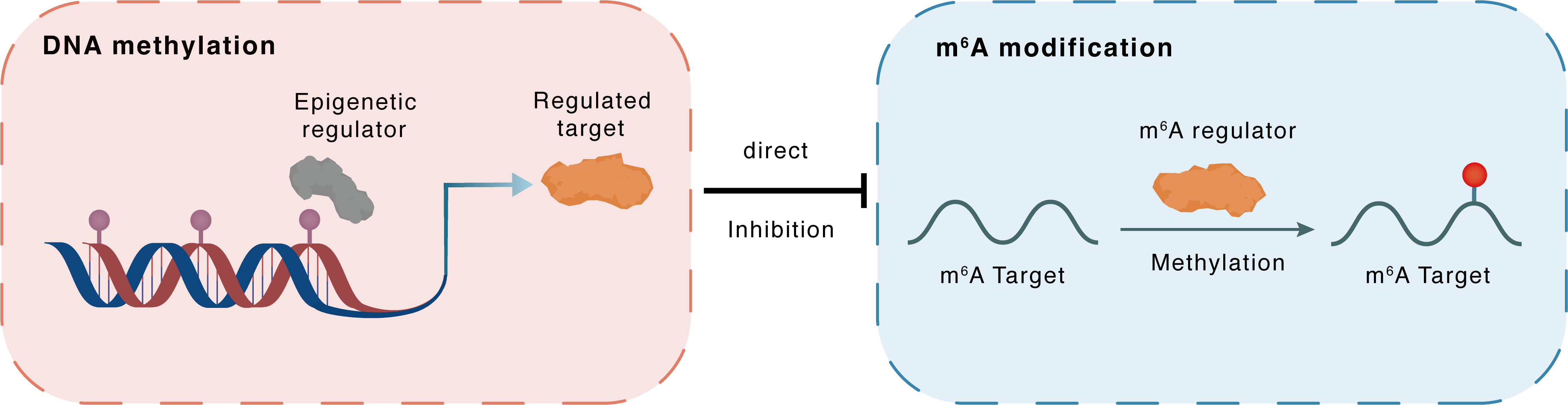 DNA methylation
DNMT1
METTL14
Direct
Inhibition
m6A modification
TMEM127
TMEM127
METTL14
Methylation
DNA methylation
DNMT1
METTL14
Direct
Inhibition
m6A modification
TMEM127
TMEM127
METTL14
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 14 (METTL14) | WRITER | |||
| m6A Target | Transmembrane protein 127 (TMEM127) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 1 (DNMT1) | WRITER | View Details | ||
| Regulated Target | Methyltransferase-like protein 14 (METTL14) | View Details | |||
| Crosstalk Relationship | DNA methylation → m6A | Inhibition | |||
| Crosstalk Mechanism | DNA methylation directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | lncRNA UCA1 recruited DNA methyltransferase (DNMT1, DNMT3A, and DNMT3B) to the METTL14 promoter region to inhibit METTL14 expression in breast cancer. N6-methyladenosine (m6A) methylation of RNA by the methyltransferase complex (MTC), with core components including METTL3-METTL14 heterodimers and Wilms' tumor 1-associated protein (WTAP), contributes to breast tumorigenesis.depletion of METTL3a globally disrupts m6A deposition, and METTL3a mediates mammalian target of rapamycin (mTOR) activation via m6A-mediated suppression of Transmembrane protein 127 (TMEM127) expression. | ||||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | |||
| Responsed Drug | Rapamycin | ||||
In-vitro Model |
MDA-MB-231 | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| T-47D | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| HEK293T | Normal | Homo sapiens | CVCL_0063 | ||
| In-vivo Model | Approximately 1 × 106 viable MDA-MB-231 breast cancer cells were resuspended in 1:1 ratio in 50 μ l medium and 50 μ l matrigel (Corning, 354234) and injected orthotopically into the fourth mammary fat pad of each mouse. After injection, tumor size was measured twice a week using an electronic caliper. Tumor volumes were calculated with the formula: volume = (L × W2)/2, where L is the tumor length and W is the tumor width measured in millimeters. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| DNA (cytosine-5)-methyltransferase 1 (DNMT1) | 27 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| SGI110 | Phase 3 | [3] | ||
| MOA | Modulator | |||
| External Link | ||||
| Guadecitabine | Phase 3 | [4] | ||
| Synonyms |
UNII-2KT4YN1DP7; 929901-49-5; 2KT4YN1DP7; SGI-110 free acid; Guadecitabine [USAN:INN]; GuadecitabineSGI-110; Guadecitabine (USAN/INN); CHEMBL3544916; Guanosine, 2'-deoxy-5-azacytidylyl-(3'-5')-2'-deoxy-; ZINC43203165; AKOS027321496; AKOS030238181; DB11918; CS-3089; HY-13542; D10877; 2'-deoxy-5-azacytidylyl-(3'-5')-2'-deoxyguanosine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CC-486 | Phase 3 | [5] | ||
| Synonyms |
AG-14361; AG14361; 328543-09-5; UNII-48N0U0K50I; AG 14361; CHEMBL65892; 48N0U0K50I; Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-; AG-014361; 1-(4-((dimethylamino)methyl)phenyl)-8,9-dihydro-2,7,9a-triazabenzo[cd]azulen-6(7H)-one; Imidazo(4,5,1-jk)(1,4)benzodiazepin-7(4H)-one, 2-(4-((dimethylamino)methyl)phenyl)-5,6-dihydro-; 2-[4-[(Dimethylamino)methyl]phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one; SMR000486393; MLS006011157; MLS001065917; Nucleoside analogue
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| S-110 | Phase 3 | [6] | ||
| Synonyms |
DNA demethylating agent (myelodysplastic syndrome), Supergen
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Palifosfamide | Phase 2 | [7] | ||
| Synonyms |
ZIO-201
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RX-3117 | Phase 2 | [8] | ||
| Synonyms |
Antimetabolite (cancer), Rexahn; Antimetabolite (cancer), Rexahn/ Teva
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Antroquinonol | Phase 2 | [9] | ||
| Synonyms |
Hocena; Fungal extract (cancer), Golden Biotechnology
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK4172239 | Phase 1 | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27376512-Compound-miR-155-5p | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 27.88 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example11 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 39440 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example16 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 22520 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example5 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50(DNMT1) = 3530 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-2HPE | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 135.2 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-1 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 2003 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example4 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 13810 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-2 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 434.1 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-1HPE | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 917.5 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example8 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6850 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-433 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 4.22 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example30 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27376512-Compound-MTC-424 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1940 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-427 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 295 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-422 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1430 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-423 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 363 nM | |||
| External Link | ||||
| PMX-700 | Investigative | [12] | ||
| Synonyms |
SJ-005019; SJ-005059; DC-010-116; Temozolomide analogs (cancer), Pharminox
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| XB-05 | Investigative | [12] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CP-4200 | Investigative | [12] | ||
| Synonyms |
Lipidated azacitidine (cancer, Lipid Vector), Clavis Pharma; 5-azacytidine-5'-elaidate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2C60: Breast cancer | 2 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Entrectinib | Approved | [13] | ||
| Synonyms |
1108743-60-7; RXDX-101; UNII-L5ORF0AN1I; Entrectinib (RXDX-101); L5ORF0AN1I; Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-; Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-; Entrectinib [USAN:INN]; YMX; Kinome_2659; Entrectinib(rxdx-101); Entrectinib (USAN/INN); SCHEMBL3512601; GTPL8290; CHEMBL1983268; KS-00000TSK
Click to Show/Hide
|
|||
| External Link | ||||
| Everolimus | Approved | [14] | ||
| External Link | ||||
References