m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT02053
|
[1] | |||
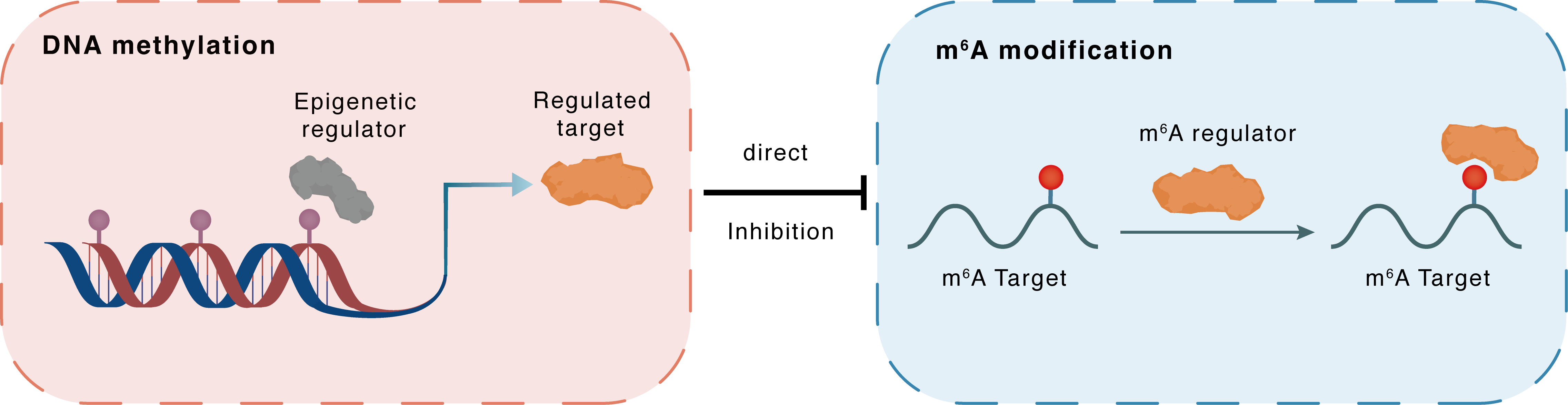 DNA methylation
DNMT1
FMR1
Direct
Inhibition
m6A modification
GPX4
GPX4
FMR1
DNA methylation
DNMT1
FMR1
Direct
Inhibition
m6A modification
GPX4
GPX4
FMR1
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Synaptic functional regulator FMR1 (FMR1) | READER | |||
| m6A Target | Phospholipid hydroperoxide glutathione peroxidase GPX4 (GPX4) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 1 (DNMT1) | WRITER | View Details | ||
| Regulated Target | Fragile X messenger ribonucleoprotein 1 (FMR1) | View Details | |||
| Crosstalk Relationship | DNA methylation → m6A | Inhibition | |||
| Crosstalk Mechanism | DNA methylation directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | USP7 was able to recruit DNMT1 to the FMR1 promoter region, which increased promoter methylation rates and suppressed FMR1 expression. TBK1 interacts with FMR1 to suppresses ferroptosis in renal tubular epithelial cells, manifested by decreased iron ion content and oxidative stress and increased cell viability and Phospholipid hydroperoxide glutathione peroxidase GPX4 (GPX4) expression level, thus alleviating I/R-induced renal injury | ||||
| Responsed Disease | Acute kidney failure | ICD-11: GB60 | |||
| Cell Process | Cell proliferation | ||||
In-vitro Model |
HK-2 [Human kidney] | Normal | Homo sapiens | CVCL_0302 | |
| In-vivo Model | All rats were housed under a 12 h light/dark cycle with constant temperature about 25 ° C and relative humidity approximating 55%. The rats had free access to food and water for 10 days prior to the experiment. After that, the renal ischemia-reperfusion injury rat model was established. SD rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (25 mg/kg). Then an incision was made in the rat abdomen, and the right kidney was removed for nephrectomy. The left kidney was exposed after a midline incision, the renal artery was clamped for 45 min with a nontraumatic clamp, and renal blood flow was then restored. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| DNA (cytosine-5)-methyltransferase 1 (DNMT1) | 27 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| SGI110 | Phase 3 | [2] | ||
| MOA | Modulator | |||
| External Link | ||||
| Guadecitabine | Phase 3 | [3] | ||
| Synonyms |
UNII-2KT4YN1DP7; 929901-49-5; 2KT4YN1DP7; SGI-110 free acid; Guadecitabine [USAN:INN]; GuadecitabineSGI-110; Guadecitabine (USAN/INN); CHEMBL3544916; Guanosine, 2'-deoxy-5-azacytidylyl-(3'-5')-2'-deoxy-; ZINC43203165; AKOS027321496; AKOS030238181; DB11918; CS-3089; HY-13542; D10877; 2'-deoxy-5-azacytidylyl-(3'-5')-2'-deoxyguanosine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CC-486 | Phase 3 | [4] | ||
| Synonyms |
AG-14361; AG14361; 328543-09-5; UNII-48N0U0K50I; AG 14361; CHEMBL65892; 48N0U0K50I; Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-; AG-014361; 1-(4-((dimethylamino)methyl)phenyl)-8,9-dihydro-2,7,9a-triazabenzo[cd]azulen-6(7H)-one; Imidazo(4,5,1-jk)(1,4)benzodiazepin-7(4H)-one, 2-(4-((dimethylamino)methyl)phenyl)-5,6-dihydro-; 2-[4-[(Dimethylamino)methyl]phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one; SMR000486393; MLS006011157; MLS001065917; Nucleoside analogue
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| S-110 | Phase 3 | [5] | ||
| Synonyms |
DNA demethylating agent (myelodysplastic syndrome), Supergen
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Palifosfamide | Phase 2 | [6] | ||
| Synonyms |
ZIO-201
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RX-3117 | Phase 2 | [7] | ||
| Synonyms |
Antimetabolite (cancer), Rexahn; Antimetabolite (cancer), Rexahn/ Teva
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Antroquinonol | Phase 2 | [8] | ||
| Synonyms |
Hocena; Fungal extract (cancer), Golden Biotechnology
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK4172239 | Phase 1 | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27376512-Compound-miR-155-5p | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 27.88 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example11 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 39440 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example16 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 22520 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example5 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50(DNMT1) = 3530 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-2HPE | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 135.2 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-1 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 2003 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example4 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 13810 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-2 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 434.1 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-1HPE | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 917.5 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example8 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6850 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-433 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 4.22 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example30 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27376512-Compound-MTC-424 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1940 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-427 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 295 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-422 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1430 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-423 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 363 nM | |||
| External Link | ||||
| PMX-700 | Investigative | [11] | ||
| Synonyms |
SJ-005019; SJ-005059; DC-010-116; Temozolomide analogs (cancer), Pharminox
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| XB-05 | Investigative | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CP-4200 | Investigative | [11] | ||
| Synonyms |
Lipidated azacitidine (cancer, Lipid Vector), Clavis Pharma; 5-azacytidine-5'-elaidate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GB60: Acute kidney failure | 4 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| BQ788 | Phase 3 | [12] | ||
| Synonyms |
(2R)-2-[[(2R)-2-amino-3-(1-methoxycarbonylindol-3-yl)propanoyl]-[(2S)-2-[[(2R,6S)-2,6-dimethylpiperidine-1-carbonyl]amino]-4,4-dimethylpentanoyl]amino]hexanoic acid; BQ 788; AC1MIWQ3; GTPL1010; SCHEMBL18429752
Click to Show/Hide
|
|||
| External Link | ||||
| EA-230 | Phase 2 | [13] | ||
| Synonyms |
503844-09-5; (2S)-2-[[2-[[(2S)-5-amino-2-[[(2S)-2-aminopropanoyl]amino]-5-oxopentanoyl]amino]acetyl]amino]-3-methylbutanoic acid; L-Valine, L-alanyl-L-glutaminylglycyl-; DTXSID80436085
Click to Show/Hide
|
|||
| External Link | ||||
| MB-102 | Phase 2 | [14] | ||
| Synonyms |
Relmapirazin; UNII-Q3UQB8PQ6H; Q3UQB8PQ6H; Relmapirazin [INN]; CHEMBL1949708; SCHEMBL16738795; N,N'-((3,6-Diamino-2,5-pyrazinediyl)dicarbonyl)bis(D-serine); D-Serine, N,N'-((3,6-diamino-2,5-pyrazinediyl)dicarbonyl)bis-; 1313706-17-0
Click to Show/Hide
|
|||
| External Link | ||||
| OPI-1002 | Phase 2 | [15] | ||
| External Link | ||||
References